Abstract
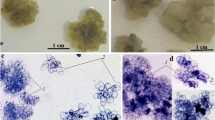
Experiments were performed to determine the effects ofcarbon source and concentration on shootmultiplication in shoot cultures of Fagussylvatica (one clone) and F. orientalis (twoclones) and on the induction of adventitious shootbuds from leaf and internode explants of F.orientalis. In general, glucose was the best carbonsource for both axillary branching and adventitiousshoot regeneration. Shoot-tip explants grown on 3–4%glucose medium produced more shoots than those onsucrose or fructose. For maximum shoot length, glucosemedium was best for two of the three clones, and 4%sucrose for the other. The number of shoots was theparameter most influenced by glucose concentration inthe adventitious shoot regeneration experiments, thenumber increasing with sugar concentration. The lowesthyperhydricity rate occurred in the presence ofsucrose in both species. Shoot growth and quality wasnegatively affected by fructose supplied media. Theuse of filter-sterilized rather than autoclavedfructose neither stimulated shoot growth nor reducedthe incidence of hyperhydricity in all three clones.The response of shoot cultures to differentcarbohydrate treatments appears to some extent to begenotype dependent.
Similar content being viewed by others
References
Barker MJ, Pijut PM, Ostry ME and Houston DR (1997) Micropropagation of juvenile and mature American beech. Plant Cell Tissue Organ Cult 51: 209–213
Belaizi M and Boxus P (1995) In vitro shoot multiplication of cork oak (Quercus suber L.). Influence of different carbohydrates. Bull Rech Agron Gembloux 30: 39–46
Bonga JM and Von Aderkas P (1992) In Vitro Culture of Trees. Dordrecht: Kluwer Academic Publishers
Chalupa V (1985) In vitro propagation of Larix, Picea, Pinus, Quercus, Fagus and other species using adenine-type cytokinins and thidiazuron. Commun Inst For Cech 14: 65–90
Chalupa V (1996) Fagus sylvatica L. (European Beech). In: Bajaj YPS (ed) Biotechnology in Agriculture and Forestry, Vol 35, Trees IV, pp 138–154. Berlin: Heidelberg: Springer-Verlag
Chauvin JE and Salesses G (1988) Effect du fructose sur la micropropagation du châtaignier Castanea sp. C R Acad Sci Paris 306 Série III: 207–212
Chevreau E, Skirvin RM, Abu-Qaoud HA, Korban SS and Sullivan JG (1989) Adventitious shoot regeneration from leaf tissue of three pear (Pyrus sp.) cultivars in vitro. Plant Cell Rep 7: 688–691
Cuenca B, Ballester A and Vieitez AM (in press) Adventitious bud regeneration from internode segments of beech. Plant Cell Tissue Organ Cult
Druart P (1995) C-source and growth response of Prunus glandulosa 'sinensis' Thund. and Malus pumila Mill. M 26 andM9 clone 29 during in vitro propagation. Bull Rech Agron Gembloux 30: 29–37
Gruselle R, Nicaise C and Boxus P (1995) Regulation of in vitro shoot multiplication in Persian walnut by different carbon sources and by ammonium phosphate. Bull Rech Agron Gembloux 30: 47–53
Harada H and Murai Y (1996) Micropropagation of Prunus mume. Plant Cell Tissue Organ Cult 46: 265–267
Hsia C-N and Korban SS (1996a) Factors affecting in vitro establishment and shoot proliferation of Rosa hybrida L. and Rosa chinensis minima. In Vitro Cell Dev Biol-Plant 32: 217–222
Hsia C-N and Korban SS (1996b) Organogenesis and somatic embryogenesis in callus cultures of Rosa hybrida and Rosa chinensis minima. Plant Cell Tissue Organ Cult 44: 1–6
Lemos EEP and Baker DA (1998) Shoot regeneration in response to carbon source on internodal explants of Annona muricata L. Plant Growth Regul 25: 105–112
Lloyd G and McCown BH (1980) Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot tip culture. Comb Proc Int Plant Propagators' Soc 30: 421–427
Meier K and Reuther G (1994) Factors controlling micropropagation of mature Fagus sylvatica. Plant Cell Tissue Organ Cult 39: 231–238
Oka S and Ohyama K (1986) Mulberry (Morus alba L.). In: Bajaj YPS (ed) Biotechnology in Agriculture and Forestry 1, Trees I, pp 384–393. Berlin, Heidelberg: Springer-Verlag
Owen HR, Wengerd D and Miller AR (1991) Culture medium pH is influenced by basal medium, carbohydrate source, gelling agent, activated charcoal and medium storage method. Plant Cell Rep 10: 583–584
Ribaux M, O'Rourke J, Gavillet S and Moncousin C (1995) Carbohydrate absorption during the in vitro proliferation of Malus EM IX. Bull Rech Agron Gembloux 30: 103–111
Romano A, Noronha C and Martins-Louçao MA (1995) Role of carbohydrates in micropropagation of cork oak. Plant Cell Tissue Organ Cult 40: 159–167
Short KC and Warburton J (1987) In vitro hardening of cultured cauliflower and chrysanthemum plantlets to humidity. Acta Hortic 212: 329–334
Tang D, Ishii K and Ohba K (1996) In vitro regeneration of Alnus cremastogyne Burk from epicotyl explants. Plant Cell Rep 15: 658–661
Thompson MR and Thorpe TA (1987) Metabolic and nonmetabolic roles of carbohydrates. In: Bonga JM and Durzan DJ (eds) Cell and Tissue Culture in Forestry, Vol 1, General Principles and Biotechnology, pp 89–112. Dordrecht: Martinus Nijhoff Publishers
Uosukainen M and Vasara T (1995) Effect of autoclaving on tissue culture medium. Bull Rech Agron Gembloux 30: 9–20
Vieitez AM, Ferro EM and Ballester A (1993) Micropropagation of Fagus sylvatica L. In Vitro Cell Dev Biol 29P: 183–188
Vieitez AM and San-José MC (1996) Adventitious shoot regeneration from Fagus sylvatica leaf explants in vitro. In Vitro Cell Dev Biol-Plant 32: 140–147
Welander M, Welander NT and Brackman A-S (1989) Regulation of in vitro shoot multiplication in Syringa, Alnus and Malus by different carbon sources. J Hort Sci 64: 361–366
Welander M and Pawlicki N (1994) Carbon compounds and their influence on in vitro growth and organogenesis. In: Lumsden PJ, Nicholas JR and Davies WJ (eds) Physiology, Growth and Development of Plants in Culture, pp 83–93. Dordrecht: Kluwer Academic Publishers
Yu X and Reed BM (1993) Improved shoot multiplication of mature hazelnut (Corylus avellana L.) in vitro using glucose as a carbon source. Plant Cell Rep 12: 256–259
Zimmermann MH and Ziegler H (1975) List of sugars and sugar alcohols in sieve-tube exudates. In: Zimmermann MH and Milburn JA (eds) Encyclopedia of Plant Physiology. New Series Vol 1, Transport in Plants I, pp 480–503. Berlin, Heidelberg, New York: Springer-Verlag
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cuenca, B., Vieitez, A. Influence of carbon source on shoot multiplication and adventitious bud regeneration in in vitro beech cultures. Plant Growth Regulation 32, 1–12 (2000). https://doi.org/10.1023/A:1006329510280
Issue Date:
DOI: https://doi.org/10.1023/A:1006329510280