Abstract
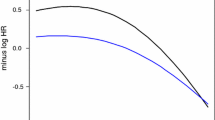
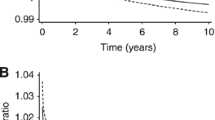
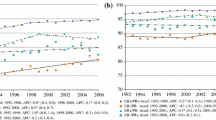
Some prognostic factors, such as steroid receptors, appear strongly related to outcome in early studies with short follow-up, but as follow-up matures the relationships appear to weaken. We investigated this phenomenon for several factors (tumor size, axillary lymph nodes, S-phase fraction, estrogen receptor (ER) status, and adjuvant therapy) in a large sample of breast cancer cases (N=2,873) with up to 17 years of follow-up for disease-free survival (DFS). Subjects in the study were identified from patients who had hormone receptor assays performed in our laboratory. Analysis of DFS included fitting a multivariate Cox proportional hazards model, testing for nonproportionality, and examining diagnostic plots. The assumption of proportional hazards was violated for several factors including ER, tumor size, and S-phase fraction. For ER, the hazard ratio was initially less than 1.0, indicating a good effect on prognosis, but increased at later times to values greater than 1.0, indicating a bad effect on prognosis. In contrast, the hazard ratios for tumor size and S-phase were initially high and decreased asymptotically toward 1.0 over time. Analysis of p53 expression in a subset of cases yielded qualitatively similar results. We conclude that several standard prognostic factors (ER, tumor size, S-phase fraction) and possibly other investigational factors have important but nonproportional effects on hazard. It is likely that violation of proportional hazards is common and not limited to breast cancer. Failure to recognize violations of proportional hazards can lead to both over- and under-estimation of the effects of important prognostic factors.
Similar content being viewed by others
References
Knight WA III, Livingston RB, Gregory EJ, McGuire WL: Estrogen receptor as an independent prognostic factor for early recurrence in breast cancer. Cancer Res 37:4669-4671, 1977
Raemaekers JMM, Beex LVAM, Koenders AJM, Pieters GFFM, Smals AGH, Benraad TJ, Kloppenborg PWC, The Breast Cancer Group: Disease-free interval and estrogen receptor activity in tumor tissue of patients with primary breast cancer: analysis after long-term follow-up. Breast Cancer Res Treat 6: 123-130, 1985
Andry G, Suciu S, Pratola D, Sylvester R, Leclercq G, Mendes de Costa P, Legros N, Andry-T'Hoot M, Verhest A, Mattheim W, Heuson J-C: Relation between estrogen receptor concentration and clinical and histological factors: Their relative prognostic importance after radical mastectomy for primary breast cancer. Eur J Cancer Clin Oncol 25:319-329, 1989
Cox DR: Regression models and life-tables (with discussion). J R Statist Soc B 34:187-200, 1972
Gray RJ: Flexible methods for analyzing survival data using splines, with application to breast cancer prognosis. J Am Statist Assoc 87:942-951, 1992
Gray RJ: Spline-based tests in survival analysis. Biometrics 50:640-652, 1994
Clark GM, Dressler LG, Owens MA, Pounds G, Oldaker T, McGuire WL: Prediction of relapse or survival in patients with node-negative breast cancer by DNA flow cytometry. N Engl J Med 320:627-633, 1989
McGuire WL, De La Garza M, Chamness GC: Evaluation of estrogen receptor assays in human breast cancer tissue. Cancer Res 37:637-639, 1977
Powell B, Garola RE, Chamness GC, McGuire WL: Measurement of progesterone receptor in human breast cancer biopsies. Cancer Res 39:1678-1682, 1979
Dressler LG, Seamer LC, Owens MA, Clark GM, McGuire WL: DNA flow cytometry and prognostic factors in 1331 frozen breast cancer specimens. Cancer 61:420-427, 1988
Wenger CR, Beardslee S, Owens MA, Pounds G, Oldaker T, Vendely P, Pandian MR, Harrington D, Clark GM, McGuire WL: DNA ploidy, S-phase, and steroid receptors in more than 127,000 breast cancer patients. Breast Cancer Res Treat 28:9-20, 1993
Allred DC, Clark GM, Elledge R, Fuqua SA, Brown RW, Chamness GC, Osborne CK, McGuire WL: Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in nodenegative breast cancer. J Natl Cancer Inst 85:200-206, 1993
Allred DC, Clark GM, Tandon AK, McGuire WL: Immunohistochemistry on histological sections from small (50 mg) samples of pulverized breast cancer. J Histotech 16:117-120, 1993
Grambsch PM, Therneau TM: Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81:515-526, 1994
Schoenfeld D: Chi-squared goodness of fit tests for the proportional hazards regression model. Biometrika 67:145-153, 1980
Statistical Sciences: S-PLUS Version 3.3 Supplement. StatSci (a division of MathSoft, Inc.), Seattle, 1995
Hess KR: Graphical methods for assessing violations of the proportional hazards assumptions in Cox regression. Stat Med 14:1707-1723, 1995
Therneau T: SURVIVAL4.1 available through Stat-Lib (http://lib.stat.cmu.edu/). Mayo Foundation for Education and Research, Rochester MN, 1994
Altman DG, De Stavola BL, Love SB, Stepniewska KA: Review of survival analyses published in cancer journals. Br J Cancer 72:511-518, 1995
Concato J, Feinstein AR, Holford TR: The risk of determining risk with multivariable models. Ann Int Med 118:201-210, 1993
Gore SM, Pocock SJ, Kerr GR: Regression models and non-proportional hazards in the analysis of breast cancer survival. Appl Statist 33:176-195, 1984
Ulm K, Jänicke F, Pache L, Dannegger F: Change in prognostic impact of uPA and PAI-1 in node-negative breast cancer patients during the follow-up. Breast Cancer Res Treat 37(S):59, 1996
Wei LJ: The accelerated failure time model: a useful alternative to the Cox regression model in survival analysis. Stat Med 11:1871-1879, 1992
Bennett S: Log-logistic regression models for survival data. Appl Statist 32:165-171, 1983
Collett D: Modelling Survival Data in Medical Research. Chapman & Hall, New York, 1994
Rights and permissions
About this article
Cite this article
Hilsenbeck, S.G., Ravdin, P.M., de Moor, C.A. et al. Time-dependence of hazard ratios for prognostic factors in primary breast cancer. Breast Cancer Res Treat 52, 227–237 (1998). https://doi.org/10.1023/A:1006133418245
Issue Date:
DOI: https://doi.org/10.1023/A:1006133418245