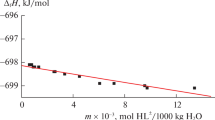
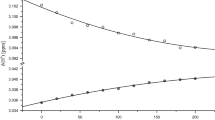
Abstract
The densities and heat capacity ratios of aqueous solutions of c-glycylglycine,c-alanylalanine, and c-sarcosylsarcosine have been measured at 15, 25, 40, and55 °C. These data have been used to calculate apparent molar volumes andapparent molar heat capacities, which, in turn, have been used in the calculationof partial molar properties at infinite dilution. The merits of using thesethermodynamic data in the calculation of glycyl group and alanine side chain groupcontributions are discussed with respect to the modeling of the thermodynamicproperties of denatured proteins in aqueous solution. The study concludes thatc-glycylglycine may not be the most suitable model compound from which toobtain the thermodynamic properties of the glycyl group. However, it alsoindicates that the cyclic dipeptides merit further investigation with respect to thethermodynamic properties of protein side chains.
Similar content being viewed by others
REFERENCES
E. J. Cohn and J. T. Edsall, Proteins, Amino Acids and Peptides (Hafner, New York, 1965).
M. Iqbal and R. E. Verrall, J. Biol. Chem. 263, 4159 (1988).
M. Häckel, H. J. Hinz, and G. R. Hedwig, Thermochim. Acta 308, 23 (1998).
M. Häckel, H. J. Hinz, and G. R. Hedwig, Biophys. Chem. 73, 163 (1998).
T. Vogl, H-J. Hinz, and G. R. Hedwig, Biophys. Chem. 54, 261 (1995).
G. R. Hedwig, J. Chem. Soc. Faraday Trans. 89, 2761, (1993).
J. F. Reading and G. R. Hedwig, J. Chem. Soc. Faraday Trans. 86, 3117 (1990).
G. I. Makhatadze and P. L. Privalov, J. Mol. Biol. 213, 375 (1990).
G. I. Makhatadze and P. L. Privalov, In Physical Properties of Polymers Handbook, J. E. Mark, ed. (Academic Press, New York, 1996), Chap. 8.
P. L. Privalov, E. I. Tiktopulo, S. Yu. Venyaminov, Yu. V. Griko, G. I. Makhatadze, and N. N. Khechinashvili, J. Mol. Biol. 205, 737 (1989).
P. L. Privalov and G. I. Makhatadze, J. Mol. Biol. 213, 385 (1990).
K. P. Murphy and S. J. Gill, J. Chem. Thermodyn. 21, 903 (1989).
A. H. Sijpkes, G. J. van de Kleut, and S. J. Gill, Biophys. Chem. 52, 75 (1994).
L. Abate, B. Palecz, C. Giancola, and G. Della Gatta, J. Chem. Thermodyn. 29, 359 (1997).
R. A. Marriott, A. W. Hakin, and J. L. Liu, J. Solution Chem. 27, 771 (1998).
M. M. Duke, A. W. Hakin, R. M. McKay, and K. E. Preuss, Can. J. Chem. 72, 1489 (1994).
G. S. Kell, J. Chem. Eng. Data 12, 66 (1967).
J. E. Desnoyers, C. De Vissier, G. Perron, and P. Picker, J. Solution Chem. 5, 605 (1976).
G. S. Kell, Water-A Comprehensive Treatise, Vol. 1, F. Franks, ed. (Plenum Press, New York, 1972).
A. W. Hakin, M. M. Duke, S. A. Klassen, R. M. McKay, and K. E. Preuss, Can. J. Chem. 72, 362 (1994).
G. I. Makhatadze, S. J. Gill, and P. L. Privalov, Biophys. Chem. 38, 33 (1990).
S. Cabani, P. Gianni, V. Mollica and L. Lepori, J. Solution Chem. 10, 563 (1981).
A. K. Mishra and J. C. Ahluwalia, J. Phys. Chem. 88, 86 (1984).
S. Cabani, G. Conti, E. Matteoli, and M. R. Tiné J. Chem. Soc. Faraday Trans. 1 77, 2385 (1981).
F. Shahidi and P. G. Farrell, J. Chem. Soc. Faraday Trans. 1, 74, 1268 (1978).
G. I. Makhatadze, V. N. Medvedkin, and P. L. Privalov, Biopolymers 30, 1001 (1990).
T. V. Chalikian, A. P. Sarvazyan, T. Funck, and K. J. Breslauer, Biopolymers 34, 541 (1994).
S. Cabani, G. Conti, and E. Matteoli, Biopolymers 16, 465 (1977).
G. R. Hedwig, private communication (1999).
M. Häckel, H-J. Hinz, and G. R. Hedwig, Science, J. Mol. Biol. 291, 197 (1999).
Y. Maham, L. G. Hepler, A. E. Mather, A. W. Hakin, and R. A. Marriott, J. Chem. Soc. Faraday Trans. 93, 1747 (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hakin, A.W., Kowalchuck, M.G., Liu, J.L. et al. Thermodynamics of Protein Model Compounds: Apparent and Partial Molar Heat Capacities and Volumes of Several Cyclic Dipeptides in Water. Journal of Solution Chemistry 29, 131–151 (2000). https://doi.org/10.1023/A:1005169114258
Issue Date:
DOI: https://doi.org/10.1023/A:1005169114258