Abstract
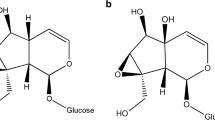
The Glanville fritillary butterfly Melitaea cinxia feeds upon two host plant species in Å land, Finland, Plantago lanceolataand Veronica spicata, both of which produce iridoid glycosides. Iridoids are known to deter feeding or decrease the growth rate of many generalist insect herbivores, but they often act as oviposition cues to specialist butterflies and are feeding stimulants to their larvae. In this study, two iridoid glycosides (aucubin and catalpol) were analyzed by micellar electrokinetic capillary chromatography. We measured the spatial and temporal variation of iridoid glycosides in natural populations of the host plants of M. cinxia. We also analyzed the aucubin and catalpol content in plants in relation to their use by ovipositing females, and in relation to the incidence of parasitism of M. cinxia larvae in natural populations. The mean concentrations of aucubin and catalpol were higher in P. lanceolata than in V. spicata, and catalpol concentrations were higher than aucubin concentrations in both host species. Plantago lanceolata individuals that were used for oviposition by M. cinxia had higher aucubin concentrations than random plants and neighboring plants. Additionally, oviposition and random plants had higher catalpol concentrations than neighboring plants, indicating that ovipositing females select for high iridoid glycoside plants or that oviposition induces iridoid glycoside production in P. lanceolata. Parasitism by the specialist parasitoid wasp Cotesia melitaearum occurred most frequently in larval groups that were feeding on plants with low concentrations of catalpol, irrespective of year, population, and host plant species. Therefore, parasitoids appear to avoid or perform poorly in host larvae with high catalpol content.
Similar content being viewed by others
References
Adler, L. S., Schmitt, J., and Bowers, M. D. 1995. Genetic variation in defensive chemistry in Plantago lanceolata (Plantaginaceae) and its effect on the specialist herbivore Junonia coenia (Nymphalidae). Oecologia 101:75–85.
Agrawal, A. 2000. Plant defense: signals in insect eggs. TREE 15:357.
Awmack, C. S. and Leather, S. R. 2002. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 47:817–844.
Barbosa, P., Saunders, J. A., Kemper, J., Trumble, R., Olechno, J., and Martinat, P. 1986. Plant allelochemicals and insect parasitoids: effects of nicotine on Cotesia congregata (Say) (Hymenoptera: Braconidae) and Hyposoter annulipes (Cressen) (Hymenoptera: Ichneumonidae). J. Chem. Ecol. 12:1319–1328.
Belofsky, G., Bowers, M. D., Janzen, S., and Stermitz, F. 1989. Iridoid glycosides of Aureolaria flava and their sequestration by Euphydryas phaeton butterflies. Phytochemistry 28:1601–1604.
Bernays, E. and De Luca, C. 1981. Insect antifeedant properties of an iridoid glycoside: ipolamiide. Experientia 37:1289–1290.
Bianco, A. 1990. The chemistry of iridoids, pp. 439–497, in Atta-ur-Rahman (ed.). Studies in Natural Products Chemistry, Vol. 7. Elsevier Science, Amsterdam.
Boros, C. A. and Stermitz, F. R. 1990. Iridoids. An updated review. Part I. J. Nat. Prod. 53:1055–1147.
Boros, C. A. and Stermitz, F. R. 1991. Iridoids. An updated review. Part II. J. Nat. Prod. 51:1173–1246.
Bos, M., Harmens, H., and Vrieling. K. 1986. Gene flow in Plantago I. Gene flow and neighbourhood size in P. lanceolata. Heredity 56:43–54.
Bowers, M. D. 1980. Unpalatability as a defense strategy of Euphydryas phaeton (Lepidoptera: Nymphalidae). Evolution 34:586–600.
Bowers, M. D. 1983. The role of iridoid glycosides in host-plant specificity of checkerspot butterflies. J. Chem. Ecol. 9:475–493.
Bowers, M. D. 1984. Iridoid glycosides and host-plant specificity in larvae of the buckeye butterfly, Junonia coenia (Nymphalidae). J. Chem. Ecol. 10:1567–1577.
Bowers, M. D. 1991. Iridoid glycosides, pp. 297–325, in G. A. Rosenthal and M. R. Berenbaum (Eds.). Herbivores: Their Interactions With Secondary Plant Metabolites. Vol. 1: The Chemical Participants. 2nd ed. Academic Press, San Diego, California.
Bowers, M. 1992. The evolution of unpalatability and the cost of chemical defense in insects, pp. 216–244, in B. D. Roitberg and M. B. Isman (Eds.). Insect Chemical Ecology: An Evolutionary Approach. Chapman and Hall, New York.
Bowers, M. D. and Collinge, S. K. 1992. Fate of iridoid glycosides in different life stages of the buckeye, Junonia coenia (Lepidoptera: Nymphalidae). J. Chem. Ecol. 18:817–831.
Bowers, M. D. and Farley, S. 1990. The behaviour of grey jays, Perisoreus canadensis, towards palatable and unpalatable Lepidoptera. Anim. Behav. 39:699–705.
Bowers, M. D. and Puttick, G. M. 1986. Fate of ingested iridoid glycosides in lepidopteran herbivores. J. Chem. Ecol. 12:169–178.
Bowers, M. D. and Puttick, G. M. 1988. Response of generalist and specialist insects to qualitative allelochemical variation. J. Chem. Ecol. 14:319–334.
Bowers, M. D. and Stamp, N. E. 1992. Chemical variation within and between individuals of Plantago lanceolata (Plantaginaceae). J. Chem. Ecol. 18:985–995.
Bowers, M. D. and Stamp, N. E. 1993. Effects of plant age, genotype, and herbivory on Plantago performance and chemistry. Ecology 74:1778–1791.
Bowers, M. D., Collinge, S. K., Gamble, S. E., and Schmitt, J. 1992. Effects of genotype, habitat, and seasonal variation on iridoid glycoside content of Plantago lanceolata (Plantaginaceae) and the implications for insect herbivores. Oecologia 91:201–207.
Camara, M. D. 1997a. Physiological mechanisms underlying the costs of chemical defense in Junonia coenia Hübner (Nymphalidae): A gravimetric and quantitative genetic analyzis. Evol. Ecol. 11:451–469.
Camara, M. D. 1997b. A recent host range expansion in Junonia coenia Hübner (Nymphalidae): oviposition preference, survival, growth, and chemical defense. Evolution 51:873–884.
Camara, M. D. 1997c. Predator responses to sequestered plant toxins in buckeye caterpillars: Are tritrophic interactions locally variable? J. Chem. Ecol. 23:2093–2106.
Campbell, B. C. and Duffey, S. F. 1979. Tomatine and parasitic wasps: Potential incompatibility of plant antibiosis with biological control. Science 205:700–702.
Campbell, B. C. and Duffey, S. F. 1981. Alleviation of a-tomatine-induced toxicity to the parasitoid Hyposoter exiguae, by phytosterols in the diet of the host, Heliothis zea. J. Chem. Ecol. 7:927–946.
Damtoft, S., Jensen, S. R., and Nielsen, B. J. 1983. The biosynthesis of iridoid glucosides from 8-epi-deoxyloganic acid. Biochem. Soc. Trans. 11:593–594.
Damtoft, S., Franzyk, H., and Jensen, S. R. 1997. Iridoid glycosides from Picconia excelsa. Phytochemistry 45:743–750.
Darrow, K. and Bowers, M. D. 1997. Phenological and population variation in iridoid glycosides of Plantago lanceolata (Plantaginaceae). Biochem. Syst. Ecol. 25:1–11.
Darrow, K. and Bowers, M. D. 1999. Effects of herbivore damage and nutrient concentration on induction of iridoid glycosides in Plantago lanceolata. J. Chem. Ecol. 25:1427–1440.
Dyer, L. A. 1995. Tasty generalists and nasty specialists? A comparative study of antipredator mechanisms in tropical lepidopteran larvae. Ecology 76:1483–1496.
Dyer, L. A. and Bowers, M. D. 1996. The importance of sequestered iridoid glycosides as a defense against an ant predator. J. Chem. Ecol. 22:1527–1539.
El-Naggar, L. J. and Beal, J. L. 1980. Iridoids. A review. J. Nat. Prod. 43:649–707.
Fajer, E. D., Bowers, M. D., and Bazzaz, F. A. 1992. The effect of nutrients and enriched CO2 environments on production of carbon-based allelochemicals in Plantago: a test of the carbon/nutrient balance hypothesis. Am. Nat. 140:707–723.
Gange, A. C. and West, H. M. 1994. Interactions between arbuscular mycorrhizal fungi and foliar-feeding insects in Plantago lanceolata L. New Phytol. 128:79–87.
Gardner, R. D. and Stermitz, F. R. 1988. Host-plant utilization and iridoid glycoside sequestration by Euphydryas anicia (Lepidoptera: Nymphalidae). J. Chem. Ecol. 14:2147–2168.
Hanski, I. 1999. Metapopulation Ecology. Oxford University Press, New York.
Herms, D. A. and Mattson, W. J. 1992. The dilemma of plants: to grow or to defend. Q. Rev. Biol. 67:293–335.
Inouye, H. 1991. Iridoids, pp. 99–143, in P. M. Dey and J. B. Harborne (Eds.). Methods in Plant Biochemistry, Vol. 7. Academic Press, San Diego, California.
Jones, C. G. and Firn, R. D. 1991. On the evolution of plant secondary chemical diversity. Phil. Trans. R. Soc. London B 333:273–280.
Judd, W. S., Campbell, C. S., Kellogg, E. A., and Stevens, P. F. 1999. Plant Systematics: A Phylogenetic Approach. Sinauer, Sunderland, Massachusetts.
Koricheva, J., Larsson, S., and Haukioja, E. 1998. Insect performance on experimentally stressed woody plants: A meta-analyzis. Annu. Rev. Entomol. 43:195–216.
Kuussaari, M., Singer, M., and Hanski, I. 2000. Local specialization and landscape-level influence on host use in an herbivorous insect. Ecology 81:2177–2187.
Lei, G.-C. and Camara, M. D. 1999. Behaviour of a specialist parasitoid, Cotesia melitaearum: from individual behaviour to metapopulation processes. Ecol. Entomol. 24:59–72.
Lei, G.-C. and Hanski, I. 1997. Metapopulation structure of Cotesia melitaearum, a specialist parasitoid of the butterfly Melitaea cinxia. Oikos 78:91–100.
Lei, G.-C., Vikberg, V., Nieminen, M., and Kuussaari, M. 1997. The parasitoid complex attacking Finnish populations of the Glanville fritillary Melitaea cinxia (Lep: Nymphalidae), an endangered butterfly. J. Nat. Hist. 31:635–648.
L'Empereur, K. M. and Stermitz, F. R. 1990a. Iridoid glycoside content of Euphydryas anicia (Lepidoptera: Nymphalidae) and its major hostplant, Besseya plantaginea (Scrophulariaceae), at a high plains Colorado site. J. Chem. Ecol. 16:187–197.
L'Empereur, K. M. and Stermitz, F. R. 1990b. Iridoid glycoside metabolism and sequestration by Poladryas minuta (Lepidoptera: Nymphalidae) feeding on Penstemon virgatus (Scrophulariaceae). J. Chem. Ecol. 16:1495–1506.
Marak, H. B., Biere, A., and Damme, J. M. M. 2000. Direct and correlated responses to selection on iridoid glycosides in Plantago lanceolata L. J. Evol. Biol. 13:985–996.
Nieminen, M., Siljander, M., and Hanski, I. 2003. Structure and dynamics of Melitaea cinxia metapopulations, In P. R. Ehrlich and I. Hanski (Eds.). On the Wings of Checkerspots. Oxford University Press, New York. In press.
Olmstead, R. G., dePamphilis, C. W., Wolfe, A. D., Young, N. D., Elisons, W. J., and Reeves, P. A. 2001. Disintegration of the Scrophulariaceae. Am. J. Bot. 88:348–361.
Pereyra, P. C. and Bowers, M. D. 1988. Iridoid glycosides as oviposition stimulants for the buckeye butterfly, Junonia coenia (Nymphalidae). J. Chem. Ecol. 14:917–928.
Puttick, G. M. and Bowers, M. D. 1988. Effect of qualitative and quantitative variation in allelochemicals on a generalist insect: Iridoid glycosides and the southern armyworm. J. Chem. Ecol. 14:335–351.
Stamp, N. 1992. Relative susceptibility to predation of two species of caterpillars on plantain. Oecologia 92:124–129.
Stamp, N. E. and Bowers, M. D. 1994. Effects of cages, plant age and mechanical clipping on plantain chemistry. Oecologia 99:66–71.
Stamp, N. E. and Bowers, M. D. 2000. Do enemies of herbivores influence plant growth and chemistry? Evidence from a seminatural experiment. J. Chem. Ecol. 26:2367–2386.
Stephenson, A. G. 1981. Toxic nectar deters nectar thieves of Catalpa speciosa. Am. Midl. Nat. 105:381–383.
Stephenson, A. G. 1982. Iridoid glycosides in the nectar of Catalpa speciosa are unpalatable to nectar thieves. J. Chem. Ecol. 8:1025–1034.
Stermitz, F. R., Gardner, D. R., Odendaal, F. J., and Ehrlich, P. R. 1986. Euphydryas anicia (Lepidoptera: Nymphalidae) utilization of iridoid glycosides from Castilleja and Besseya (Schrophulariaceae) host plants. J. Chem. Ecol. 12:1459–1468.
Suomi, J., Sirén, H., Hartonen, K., and Riekkola, M.-L. 2000. Extraction of iridoid glycosides and their determination by micellar electrokinetic capillary chromatography. J. Chromatogr. A 868:73–83.
Suomi, J., Sirén, H., Wiedmer, S. K., and Riekkola, M.-L. 2001. Isolation of aucubin and catalpol from Melitaea cinxia larvae and quantification by micellar electrokinetic capillary chromatography. Anal. Chim. Acta 429:91–99.
Teramura, A. H. 1983. Experimental ecological genetics in Plantago IX. Differences in growth and vegetative reproduction in Plantago lanceolata L. (Plantaginaceae) from adjacent habitats. Am. J. Bot. 70:53–58.
Theodoratus, D. H. and Bowers, M. D. 1999. Effects of sequestered iridoid glycosides on prey choice of the prairie wolf spider, Lycosa carolinensis. J. Chem. Ecol. 25:283–295.
Thorpe, K. W. and Barbosa, P. 1986. Effects of consumption of high and low nicotine tobacco by Manduca sexta (Lepidoptera: Sphingidae) on the survival of gregarious endoparasitoid Cotesia congregata. J. Chem. Ecol. 12:1329–1337.
van Nouhuys, S. and Hanski, I. 1999. Host diet affects extinctions and colonizations in a parasitoid metapopulation. J. Anim. Ecol. 68:1248–1258.
van Nouhuys, S. and Hanski, I. 2002. Multitrophic interactions in space: metacommunity dynamics in fragmented landscapes, pp. 124–147, in T. Tscharntke and B. A. Hawkins (Eds.). Multitrophic Level Interactions. Cambridge University Press, Cambridge.
van Nouhuys, S. and Hanski, I. 2003. Natural enemies of checkerspot butterflies, In P. R. Ehrlich and I. Hanski (Eds.). On the Wings of Checkerspots. Oxford University Press, New York, In press.
van Tienderen, P. H. 1992. Variation in a population of Plantago lanceolata along a topographical gradient. Oikos 64:560–572.
Zangerl, A. R. and Berenbaum, M. R. 1993. Plant chemistry, insect adaptations to plant chemistry, and host plant utilization patterns. Ecology 74:47–54.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nieminen, M., Suomi, J., Van Nouhuys, S. et al. Effect of Iridoid Glycoside Content on Oviposition Host Plant Choice and Parasitism in a Specialist Herbivore. J Chem Ecol 29, 823–844 (2003). https://doi.org/10.1023/A:1022923514534
Issue Date:
DOI: https://doi.org/10.1023/A:1022923514534