Abstract
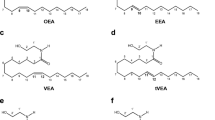
The aim of this study was to isolate a compound from blood plasma that inhibits intestinal diarrhea and that appears also to regulate fluid volumes in other organs. The isolation procedure included lipid extraction, liquid chromatography, and gas chromatography. The active substance was identified by mass spectrometry as erucamide (MW 337 Da). The biological effect was reproduced with authentic erucamide. Erucamide is a fatty acid amide, such as oleamide and anandamide, which modulate other physiological functions in a receptor-mediated fashion. All the exact biological functions of erucamide are as yet to be defined, but it is already known to stimulate angiogenesis. Erucamide concentrations were determined in body organs from the pig. The blood plasma level was 3 ng/g, and those of lung, kidney, liver, and brain were 12, 2.5, 1.0, and 0.5 ng/g, respectively. Erucamide was below detection level in the intestine, but is known to be present in the cerebrospinal fluid. In the rat, 3H-erucamide was accumulated in vivo into lung, liver, and spleen and in vitro into lung, liver, brain, and intestine. The in vitro uptake was time and temperature dependent, but not saturable.
Similar content being viewed by others
REFERENCES
Venero, J. L., Vizuete, M. L., Ilundain, A. A., Machado, A., Echevarria, M., and Cano, J. 1999. Detailed localization of aquaporin-4 messenger RNA in the CNS: Preferential expression in periventricular organs. Neuroscience 94:239–250.
Badaut, J., Lasbennes, F., Magistretti, P. J., and Regli, L. 2002. Aquaporins in brain: Distribution, physiology, and pathophysiology. J. Cereb. Blood Flow Metab. 22:367–378.
Manley, G. T., Fujimura, M., Ma, T., et al. 2000. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 6:159–163.
Chen, H. H., Lainchbury, J. G., Harty, G. J., and Burnett, J. C., Jr. 2002. Maximizing the natriuretic peptide system in experimental heart failure: Subcutaneous brain natriuretic peptide and acute vasopeptidase inhibition. Circulation 105:999–1003.
Rosenberg, G. A. and Estrada, E. Y. 1995. Atrial natriuretic peptide blocks hemorrhagic brain edema after 4–hour delay in rats. Stroke 26:874–877.
Doczi, T. 1993. Volume regulation of the brain tissue: a survey. Acta Neurochir. (Wien.) 121:1–8.
Vajda, Z., Pedersen, M., Doczi, T., et al. 2001. Effects of centrally administered arginine vasopressin and atrial natriuretic peptide on the development of brain edema in hyponatremic rats. Neurosurgery 49:697–704; discussion 704–695.
Goto, A., Yamada, K., Yagi, N., Yoshioka, M., and Sugimoto, T. 1992. Physiology and pharmacology of endogenous digitalis-like factors. Pharmacol. Rev. 44:377–399.
Takahashi, H. 2000. Endogenous digitalis-like factor: An update. Hypertens. Res. 23:S1–S5.
Hamlyn, J. M., Blaustein, M. P., Bova, S., et al. 1991. Identification and characterization of a ouabain-like compound from human plasma. Proc. Natl. Acad. Sci. USA 88:6259–6263.
Clerico, A., Paci, A., Del Chicca, M. G., Biver, P., and Giampietro, O. 1992. Endogenous digitalis-like factors in human milk. Clin. Chem. 38:504–506.
Halperin, J. A., Martin, A. M., and Malave, S. 1985. Increased digitalis-like activity in human cerebrospinal fluid after expansion of the extracellular fluid volume. Life Sci. 37:561–566.
Halperin, J. A., Riordan, J. F., and Tosteson, D. C. 1988. Characteristics of an inhibitor of the Na+/K+ pump in human cerebrospinal fluid. J. Biol. Chem. 263:646–651.
Lorenzo, A. V., Taratuska, A., and Halperin, J. A. 1989. Suppression of cerebrospinal fluid (CSF) production by a Na+/K+ pump inhibitor extracted from human cerebrospinal fluid. Z. Kinderchir. 44:24–26.
Cloix, J. F. 1987. Endogenous digitalislike compounds: A tentative update of chemical and biological studies. Hypertension 10:I67–I70.
van der Stelt, M., Veldhuis, W. B., van Haaften, G. W., et al. 2001. Exogenous anandamide protects rat brain against acute neuronal injury in vivo. J. Neurosci. 21:8765–8771.
Bezuglov, V. V., Bobrov, M., and Archakov, A. V. 1998. Bioactive amides of fatty acids. Biochemistry (Mosc.) 63:22–30.
Johansson, C. E. 1988. The choroid plexus-arachnoid-cerebrospinal fluid system. Pages 33–104, in Boulton A, Baker G, and Walz, W. (eds.), Neuromethods, Vol VII: The neuronal microenvironment: Electrolytes and water spaces. Clifton, NJ: Humana Press.
Knuckey, N. W., Fowler, A. G., Johanson, C. E., Nashold, J. R., and Epstein, M. H. 1991. Cisterna magna microdialysis of 22Na to evaluate ion transport and cerebrospinal fluid dynamics. J. Neurosurg. 74:965–971.
Lönnroth, I. and Lange, S. 1984. Purification and characterization of a hormone-like factor which inhibits cholera secretion. FEBS Lett. 177:104–108.
Lönnroth, I., Lange, S., and Skadhauge, E. 1988. The antisecretory factors: Inducible proteins which modulate secretion in the small intestine. Comp. Biochem. Physiol. A 90:611–617.
Lange, S. and Lönnroth, I. 1984. Passive transfer of protection against cholera toxin in rat intestine. FEMS Micobiol. Lett. 24: 165–168.
Johansson, E. 1997. Cloning, expression and characterization of antisecretory factor. Department of Medical Microbiology and Immunology. University of Göteborg, Göteborg, Sweden.
Johansson, E., Lönnroth, I., Lange, S., Jonson, I., Jennische, E., and Lonnroth, C. 1995. Molecular cloning and expression of a pituitary gland protein modulating intestinal fluid secretion. J. Biol. Chem. 270:20615–20620.
Wakamatsu, K., Masaki, T., Itoh, F., Kondo, K., and Sudo, K. 1990. Isolation of fatty acid amide as an angiogenic principle from bovine mesentery. Biochem. Biophys. Res. Commun. 168: 423–429.
Mitchell, C. A., Davies, M. J., Grounds, M. D., et al. 1996. Enhancement of neovascularization in regenerating skeletal muscle by the sustained release of erucamide from a polymer matrix. J. Biomater. Appl. 10:230–249.
Arai, Y., Fukushima, T., Shirao, M., Yang, X., and Imai, K. 2000. Sensitive determination of anandamide in rat brain utilizing a coupled-column HPLC with fluorimetric detection. Biomed. Chromatogr. 14:118–124.
Lange, S. 1982. A rat model for an in vivo assay of enterotoxic diarrhea. FEMS Micobiol. Lett. 15:239–242.
Lönnroth, I. and Lange, S. 1986. Purification and characterization of the antisecretory factor: A protein in the central nervous system and in the gut which inhibits intestinal hypersecretion induced by cholera toxin. Biochim. Biophys. Acta 883:138–144.
Gecse, A., Mezei, Z., and Telegdy, G. 1986. The action of peptides and proteases on the arachidonate cascade of human and rat platelets. Adv. Exp. Med. Biol. 198:121–128.
Bertolino, F., Valentin, J. P., Maffre, M., et al. 1995. Intrinsic activity of the non-prostanoid thromboxane A2 receptor antagonist, daltroban (BM 13,505), in human platelets in vitro and in the rat vasculature in vivo. Br. J. Pharmacol. 115:210–216.
Harada, K. and Ikegami, T. 2000. Evolution of specificity in an immune network. J. Theor. Biol. 203:439–449.
Lombardi, G., Dianzani, C., Miglio, G., Canonico, P. L., and Fantozzi, R. 2001. Characterization of ionotropic glutamate receptors in human lymphocytes. Br. J. Pharmacol. 133:936–944.
Merkler, K. A., Baumgart, L. E., DeBlassio, J. L., et al. 1999. A pathway for the biosynthesis of fatty acid amides. Adv. Exp. Med. Biol. 469:519–525.
Bialer, M. 1991. Clinical pharmacology of valpromide. Clin. Pharmacokinet. 20:114–122.
Jain, M. K., Ghomashchi, F., Yu, B. Z., et al. 1992. Fatty acid amides: Scooting mode-based discovery of tight-binding competitive inhibitors of secreted phospholipases A2. J. Med. Chem. 35:3584–3586.
Cravatt, B. F., Prospero-Garcia, O., Siuzdak, G., et al. 1995. Chemical characterization of a family of brain lipids that induce sleep. Science 268:1506–1509.
Cravatt, B. F., Giang, D. K., Mayfield, S. P., Boger, D. L., Lerner, R. A., and Gilula, N. B. 1996. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83–87.
Cravatt, B. F., Demarest, K., Patricelli, M. P., et al. 2001. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl. Acad. Sci. USA 98:9371–9376.
Boger, D. L., Henriksen, S. J., and Cravatt, B. F. 1998. Oleamide: An endogenous sleep-inducing lipid and prototypical member of a new class of biological signaling molecules. Curr. Pharm. Des. 4:303–314.
Devane, W. A., Hanus, L., Breuer, A., et al. 1992. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949.
Walker, J. M., Huang, S. M., Strangman, N. M., Tsou, K., and Sanudo-Pena, M. C. 1999. Pain modulation by release of the endogenous cannabinoid anandamide. Proc. Natl. Acad. Sci. USA 96:12198–12203.
Cheer, J. F., Cadogan, A. K., Marsden, C. A., Fone, K. C., and Kendall, D. A. 1999. Modification of 5–HT2 receptor mediated behaviour in the rat by oleamide and the role of cannabinoid receptors. Neuropharmacology 38:533–541.
Mechoulam, R., Fride, E., Hanus, L., et. al. 1997. Anandamide may mediate sleep induction. Nature 389:25–26.
Guan, X., Cravatt, B. F., Ehring, G. R., et al. 1997. The sleep-inducing lipid oleamide deconvolutes gap junction communication and calcium wave transmission in glial cells. J. Cell Biol. 139:1785–1792.
Boger, D. L., Patterson, J. E., Guan, X., Cravatt, B. F., Lerner, R. A., and Gilula, N. B. 1998. Chemical requirements for inhibition of gap junction communication by the biologically active lipid oleamide. Proc. Natl. Acad. Sci. USA 95:4810–4815.
Ueda, N. and Yamamoto, S. 2000. Anandamide amidohydrolase (fatty acid amide hydrolase). Prostaglandins Other Lipid Mediat. 61:19–28.
Egertova, M., Cravatt, B. F., and Elphick, M. R. 2000. Fatty acid amide hydrolase expression in rat choroid plexus: Possible role in regulation of the sleep-inducing action of oleamide. Neurosci. Lett. 282:13–16.
Fowler, C. J., Jonsson, K. O., and Tiger, G. 2001. Fatty acid amide hydrolase: Biochemistry, pharmacology, and therapeutic possibilities for an enzyme hydrolyzing anandamide, 2–arachidonoylglycerol, palmitoylethanolamide, and oleamide. Biochem. Pharmacol. 62:517–526.
Boger, D. L., Sato, H., Lerner, A. E., et. al. 2000. Exceptionally potent inhibitors of fatty acid amide hydrolase: The enzyme responsible for degradation of endogenous oleamide and anandamide. Proc. Natl. Acad. Sci. USA 97:5044–5049.
Mendelson, W. B. and Basile, A. S. 1999. The hypnotic actions of oleamide are blocked by a cannabinoid receptor antagonist. Neuroreport 10:3237–3239.
Lange, S., Jennische, E., Johansson, E., and Lonnroth, I. 1999. The antisecretory factor: Synthesis and intracellular localisation in porcine tissues. Cell Tissue Res. 296:607–617.
Rakhshan, F., Day, T. A., Blakely, R. D., and Barker, E. L. 2000. Carrier-mediated uptake of the endogenous cannabinoid anandamide in RBL-2H3 cells. J. Pharmacol. Exp. Ther. 292: 960–967.
Beltramo, M. and Piomelli, D. 2000. Carrier-mediated transport and enzymatic hydrolysis of the endogenous cannabinoid 2–arachidonylglycerol. Neuroreport 11:1231–1235.
Bisogno, T., MacCarrone, M., De Petrocellis, L., et. al. 2001. The uptake by cells of 2–arachidonoylglycerol, an endogenous agonist of cannabinoid receptors. Eur. J. Biochem. 268:1982–1989.
Patricelli, M. P. and Cravatt, B. F. 2001. Proteins regulating the biosynthesis and inactivation of neuromodulatory fatty acid amides. Vitam. Horm. 62:95–131.
Charlton, K. M., Corner, A. H., Davey, K., Kramer, J. K., Mahadevan, S., and Sauer, F. D. 1975. Cardiac lesions in rats fed rapeseed oils. Can. J. Comp. Med. 39:261–269.
Sankhe, S. Y., Hirt, D. E., Roberts, W. P., and Havens, M. R. 1999. Evaluation of additive concentration profiles in multilayer films. Clemson, SC, USA. Annual Technical Conference—Society of Plastics Engineers, p. 2516–2520.
Hanus, L. O., Fales, H. M., Spande, T. F., and Basile, A. S. 1999. A gas chromatographic-mass spectral assay for the quantitative determination of oleamide in biological fluids. Anal. Biochem. 270:159–166.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamberger, A., Stenhagen, G. Erucamide as a Modulator of Water Balance: New Function of a Fatty Acid Amide. Neurochem Res 28, 177–185 (2003). https://doi.org/10.1023/A:1022364830421
Issue Date:
DOI: https://doi.org/10.1023/A:1022364830421