Abstract
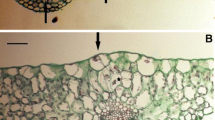
Adventitious stipular bud formation occurred in vitro in many strawberry cultivars during the proliferation phase onmedium containing Knop macronutrients, MS micronutrients, vitamins, aminoacids,2.22 μM BAP, 2.46 μM IBA and 0.29μM GA3. As described previously for cultivarGorella,cultivar Elsanta also showed adventitious stipular buds developing on theabaxial median zone between the stipule tips. To compare the shoots producedfrom both types of buds, clonal propagation was initiated from stipular budsandfrom axillary buds on the above mentioned medium. Stipular buds were separatedfrom the meristem-tip initiated plantlet and cultivated in the presence of alower BAP concentration (1.33 μM) to prevent further stipularbud formation.
During proliferation cycles, stipular originated propagules werevery easily distinguished by their specific leaf phenotype and light greencolour in comparison to plantlets cloned for an axillary bud. Theirmultiplication rate and cytokinin content were also higher than for axillarybuds. No significant difference was observed in auxin content.
Similar content being viewed by others
References
Auer C.A., Cohen J.D., Laloue M. and Cooke T.J. 1992. Comparison of benzyladenine metabolism in two Petunia hybrida lines differing in shoot org anogenesis. Plant Physiol. 98: 1035–1041.
Boxus Ph. 1974. The production of strawberry plants by in vitro micropropagation. J. Hort. Sci. 49: 209–210.
Boxus Ph. 1989. Review on strawberry mass propagation. Acta Hort. 265: 309–320.
Gatineau F., Fouche J.G., Kevers C., Hausman J.F. and Gaspar Th. 1997. Quantitative variations of indolyl compounds including IAA, IAA-aspartate and serotonin in walnut microcuttings during root induction. Biol. Plant. 39: 131–137.
George E.F. 1993. Plant Propagation by Tissue Culture. Part 1: The Technology, 573 pp.
Glauert A.M. 1975. Fixation, Dehydration, Embedding of Biological Specimens. In: Glauert A.M. (ed.), Practical Methods in Electron Microscopy. Vol. 3. North Holland Publisher Co., Amsterdam, New York, Oxford, 208 pages.
Jemmali A., Boxus Ph. and Kinet J.M. 1992. Are strawberry plantlets arising from adventitious stipule buds also true-to-type? Acta Hort. 319: 171–176.
Jemmali A., Boxus Ph., Dekegel D. and Van Heule G. 1994a. Occurrence of spontaneous, shoot regeneration on leaf stipules in relation to hyperflowering response in micropropagated strawberry plantlets in vitro. Cell Dev. Biol. 30P: 192–195.
Jemmali A. 1994. Etude physiologique et morphogénétique de la floraison chez les microplants de fraisier (Fragaria x ananassa Duch.) cv. Gorella. Thèse de Doctorat en Sc. Agro., Fac. Sc. Agro., Gembloux, Belgique.
Jemmali A., Boxus Ph., Kevers C. and Gaspar Th. 1994b. Flowering abundance of strawberry depending on the number of subcultures in vitro. Relationships with growth, rooting, peroxidase activity. In: Lumsden P.J. et al. (ed.), Physiology, Growth and Development of Plants in Culture. Kluwer Academic Publishers, Dordrecht, pp. 356–362.
Jemmali A., Boxus Ph., Kevers C. and Gaspar Th. 1995. Carryover of morphological and biochemical characteristics associated with hyperflowering of micropropagated strawberries. J. Plant Physiol. 147: 435–440.
Jemmali A., Robbe A. and Boxus Ph. 1997. Histochemical events and other fundamental differences between axillary and adventitious stipular shoots in micropropagated strawberry. Acta Hort. 439: 341–345.
Kinet J.M. and Parmentier A. 1989. The flowering behaviour of micropropagated strawberry plants cv. Gorella, the influence of the number of subcultures on the multiplication medium. Acta Hort. 265: 327–334.
Nordstrom A.C. and Eliasson L. 1991. Levels of endogenous indole-3-acetic and indole-3-acetylaspartic acid during adventitious root formation in pea cuttings. Physiol. Plant. 82: 599–605.
Rancillac M. and Nourrisseau G.J. 1987. Micropropagation and strawberry quality. Acta Hort. 265: 343–347.
Ruggini E. and Orlando R. 1992. High efficiency shoot regeneration from calluses of strawberry (Fragaria x ananassa Duch.) stipules of in vitro shoot cultures. J. Hort. Sc. 67: 577–582.
Swartz H.J., Galletta G.J. and Zimmerman R.H. 1981. Field performance and phenotype stability of tissue culture propagated strawberries. J. Amer. Soc. Hort. Sci. 106: 667–673.
Zimmerman R.H. 1981. Micropropagation of fruit plants. Acta Hort. 120: 217–222.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jemmali, A., Elloumi, N., Kevers, C. et al. Morphological and hormonal characterisation of strawberry vitroplants raised through axillary or stipular adventitious shooting. Plant Growth Regulation 38, 273–278 (2002). https://doi.org/10.1023/A:1021526808514
Issue Date:
DOI: https://doi.org/10.1023/A:1021526808514