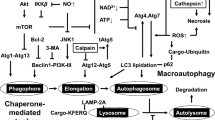
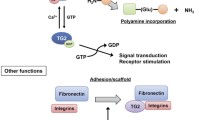
Abstract
1. One type of transglutaminase is usually accumulated in various forms of naturally occurring cell death and apoptosis. The accumulated enzyme is activated during the death process, leading to the formation of cross-linked protein structures. Degradation of the cross-linked apoptotic bodies results in the elevation of the ε(γ-glutamyl)lysine isodipeptide concentration in body fluids, which may provide a diagnostic tool to monitor the apoptosis rate in various tissues under normal and pathologic conditions.
2. Extensive protein cross-linking may be directly related to the act of killing in some cells. In others, the effect of protein cross-linking is palliative, preventing leakage of macromolecules and enhancing phagocytosis of the dead cells.
3. Tissue transglutaminase has been implicated in some physiologic functions of the nervous system.
4. The molecular machinery of apoptosis is present and easily evoked in neuronal cells.
5. Effector elements of the apoptosis process have been associated with the pathogenesis of neurologic disorders. Tissue transglutaminase, representing one of the effector elements of apoptosis, may be induced and activated in cells following ischemia. It may also participate in the formation of abnormal cell inclusions and Aβ deposits in amyloid plaques.
Similar content being viewed by others
REFERENCES
Allsopp, T. E., Wyatt, S., Paterson, H. F., and Davies, A. M. (1993). The protooncogene bcl-2 can selectively rescue neurotropic factor-dependent neurons from apoptosis. Cell 73:295–307.
Ambron, R. T., and Kremzner, L. T. (1982). Post-translational modification of neuronal proteins: evidence for transglutaminase activity in R2, the giant cholinergic neurone of Aplysia. Proc. Natl. Acad. Sci. USA 79:3442–3446.
Amendola, A., Gougeon, M.-L., Poccia, F., Bondurand, A., Fesus, L., and Piacentini, M. (1996). Tissue transglutaminase indicates high rate of apoptosis in the immune system of HIV-infected individuals. Proc. Natl. Acad. Sci. USA 93:11057–11062.
Ando, M., and Nagata, Y. (1993). Effects of depolarizing agents on transglutaminase activity in superior cervical and nodose ganglia from rats. Mol. Chem. Neuropathol. 19:121–135.
Ando, M., Tatematsu, T., Kunii, S., and Nagata, Y. (1994a). Blockade effect of nerve growth on GM1 ganglioside-induced activation of transglutaminase in superior cervical sympathetic ganglia excised from adult rat. Neurosci. Res. 19:373–378.
Ando, M., Tatematsu, T., Kusudo, S., Fujita, K., and Nagata, Y. (1994b). Possible involvement of nitric oxide in carbachol-induced activation of transglutaminase in rat superior cervical sympathetic ganglia. Neurosci. Res. 21:267–272.
Bredesen, D. E. (1994). Neuronal apoptosis: Genetic and biochemical modulation. In Tomei, L. D., and Cope, F. O. (eds), Apoptosis II: The Molecular Basis of Apoptosis in Disease, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 397–421.
Carson, D. A., and Ribeiro, J. M. (1993). Apoptosis and disease. Lancet 341:1251–1254.
Davies, A. M. (1993). Promoting motor neuron survival. Cur. Biol. 3:879–881.
Dudek, S. M., and Johnson, G. V. W. (1993). Transglutaminase catalyzes the formation of sodium dodecyl sulfate-insoluble, Alz-50-reactive polymers of τ. J. Neurochem. 61:1159–1162.
Eitan, S., and Schwartz, M. (1993). Transglutaminase that converts IL-2 into a factor cytotoxic to oligodendrocytes. Science 261:106–108.
Eitan, S., Solomon, A., Lavie, V., Yoles, E., Hirschberg, D. L., Belkin, M., and Schwartz, M. (1994). Recovery of visual response of injured adult rat optic nerve treated with transglutaminase. Science 264:1764–1768.
Ellis, H. M., Yuan, J., and Horvitz, H. R. (1991). Mechanisms and functions of cell death. Annu. Rev. Cell Biol. 7:663–698.
Facchiano, F., Benfenati, F., Valtorta, F., and Luini, A. (1993). Covalent modification of synapsin 1 by a tetanus toxin-activated transglutaminase. J. Biol. Chem. 268:4588–4591.
Fesus, L. (1993). Biochemical events in naturally occurring forms of cell death. FEBS Lett. 328:1–5.
Fesus, L. and Tarcsa, E. (1989). Formation of ɛ(γ-glutamyl)lysine isodipeptide in Chinese hamster ovary cells. Biochem. J. 263:843–849.
Fesus, L., Szucs, E. F., Barrett, K. E., Metcalfe, D. D., and Folk, J. E. (1985). Activation of transglutaminase and production of protein-bound γ-glutamylhistamine in stimulated mouse mast cells. J. Biol. Chem. 260:13771–13778.
Fesus, L., Davies, P. J. A., and Piacentini, M. (1991a). Molecular mechanisms in the program of cell death by apoptosis. Eur. J. Cell Biol. 56:170–177.
Fesus, L., Tarcsa, E., Kedei, N., Autuori, F., and Piacentini, M. (1991b). Degradation of cells dying by apoptosis leads to accumulation of ɛ(γ-glutamyl)lysine isodipeptide in culture fluid and blood. FEBS Lett. 284:109–112.
Fesus, L., Szondy, Z., and Uray, I. (1995). Probing the molecular program of apoptosis by cancer chemopreventive agents. J. Cell. Biochem. 22:151–161.
Folk, J. E. (1980). Transglutaminases. Annu. Rev. Biochem. 49:517–531.
Folk, J. E., and Finlayson, S. (1977). The ɛ(γ-glutamyl)lysine crosslink and the catalytic role of transglutaminase. Adv. Protein Chem. 31:1–133.
Friedrich, P., Fesus, L., Tarcsa, E., and Czeh, G. (1991). Protein cross-linking by transglutaminase induced in long-term potentiation in the CA1 region of hippocampal slices. Neuroscience 43:331–334.
Gentile, V., Thomazy, V., Piacentini, M., Fesus, L., and Davies, P. J. A. (1992). Transfection of tissue transglutaminase into balb-c 3T3 fibroblasts: Increased cellular adhesion fragmentation. J. Cell Biol. 119:463–474.
Gilad, G. M., and Varon, L. E. (1985). Transglutaminase activity in rat brain: Characterization, distribution and changes with age. J. Neurochem. 45:1522–1526.
Goedert, M. (1993). Tau protein and the neurofibrillary pathology of Alzheimer's disease. Trends Neurosci. 16:460–465.
Greenberg, C. S., Birckbichler, P. J., and Rice, R. H. (1992). Transglutaminases: Multifunctional crosslinking enzymes that stabilize tissues. FASEB J. 5:3071–3077.
Gschwind, M., and Huber, G. (1995). Apoptotic cell death induced by β-amyloid1–42 peptide is cell type dependent. J. Neurochem. 65:292–300.
Hand, D., Perry, M. J. M., and Haynes, L. W. (1993). Cellular transglutaminase in neural development. Int. J. Dev. Neurosci. 11:709–720.
Haynes, L. W., Perry, M. J. M, and Hand, D. (1992). A retinoid-inducible protein in developing cerebellar neurones. Biochem. Soc. Trans. 20:159S.
Héron, A., Pollard, H., Dessi, F., Moreau, J., Lasbennes, F., Ben-Ari, Y., and Charriaut-Marlangue, C. (1993). Regional variability in DNA fragmentation after global ischemia evidenced by combined histological and gel electrophoresis observations in the rat brain. J. Neurochem. 61:1973–1976.
Ikura, K., Takahata, K., and Sasaki, R. (1993). Cross-linking of a synthetic partial-length (1–28) peptide of the Alzheimer β/A4 amyloid protein by transglutaminase. FEBS Lett. 326:109–111.
Iwaki, T., Miyazono, M., Hitotsumatsu, T., and Tateishi, J. (1994). An immunohistochemical study of tissue transglutaminase in gliomas with reference to their cell dying process. Am. J. Pathol. 145:776–781.
Kim, I., Gorman, J. J., Park, S., Chung, S., and Steinert, P. (1993). The deduced sequence of the novel protransglutaminase E of human and mouse. J. Biol. Chem. 268:12682–12694.
Krajewski, S., Mai, J. K., Krajewska, M., Sikorska, M., Mossakowski, M. J., and Reed, J. C. (1995). Upregulation of bax protein levels in neurons following cerebral ischemia. J. Neurosci. 15:6364–6376.
Linnik, M. D., Zobrist, R. H., and Hatfield, M. D. (1993). Evidence supporting a role for programmed cell death in focal cerebral ischemia in rats. Stroke 24:2002–2009.
Martin, D. P., Schmidt, R. E., DiStefano, P. S., Lowry, O. H., Carter, J. G., and Johnson, E. M., Jr. (1988). Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J. Cell Biol. 106:829–844.
Martin, D. R., Ito, A., Horigome, K., Lampe, P. A., and Johnson, E. M., Jr. (1992). Biochemical characterization of programmed cell death in NGF-deprived sympathetic neurons. J. Neurobiol. 23:1205–1220.
Masu, Y., Wolf, E., Holtmann, B., Sendtner, M., Brem, G., and Thoenen, H. (1993). Disruption of the CNTF gene results in motor neuron degeneration. Nature 365:27–33.
Matesz, K., Fesus, L., Polgar, E., and Torok, Zs. (1991). Expression of tissue transglutaminase in the developing nervous system. Eur. J. Neurosci. 4S:290.
Melino, G., Annichiarico-Petruzzelli, M., Piredda, L., Candi, E., Gentile, V., Davies, P. J. A., and Piacentini, M. (1994). Tissue transglutaminase and apoptosis: Sense and antisense transfection studies in human neuroblastoma cells. Mol. Cell. Biol. 14:6584–6592.
Miller, M. L., and Johnson, G. V. W. (1995). Transglutaminase cross-linking of the τ protein. J. Neurochem. 65:1760–1770.
Nagata, S., and Golstein, P. (1995). The fas death factor. Science 267:1449–1455.
Okamoto, M., Matsumoto, M., Ohtsuki, T., Taguchi, A., Mikoshiba, K., Yanagihara, T., and Kamada, T. (1993). Internucleosomal DNA cleavage involved in ischemia-induced neuronal death. Biochem. Biophys. Res. Commun. 196:1356–1362.
Oppenheim, R. W. (1985). Naturally occurring cell death during neural development. Trends Neurosci. 17:487–493.
Oppenheim, R. W., Prevette, D., Tytell, M., and Homma, S. (1990). Naturally occurring and induced neuronal death in the chick embryo in vivo requires protein and RNA synthesis: Evidence for the role of cell death genes. Dev. Biol. 138:104–113.
Oppenheim, R. W., Qin-Wei, Y., Prevette, D., and Yan, Q. (1992). Brain-derived neurotropic factor rescues developing avian motoneurons from cell death. Nature 360:755–757.
Oppenheim, R. W., Houenou, L. J., Johnson, J. E., Lin, L.-F., Li, L., Lo, A. C., Newsome, A. L., Prevette, D. M., and Wang, S. (1995). Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature 373:344–346.
Osborne, A. B., and Schwartz, L. M. (1994). Essential genes that regulate apoptosis. Trends Cell Biol. 4:394–399.
Paschen, W., Röhn, G., and Schmidt-Kastner, R. (1990). Transglutminase activity in reversible cerebral ischemia in the rat. Neurosci. Lett. 110:232–236.
Pastuszko, A., Wilson, D. F., and Erecinska, M. (1986). A role of transglutaminase in neurotransmitter release by rat brain synaptosomes. J. Neurochem. 46:599–508.
Piacentini, M., Martinet, N., Beninati, S., and Folk, J. A. (1988). Free and protein-conjugated polyamines in mouse epidermal cells. J. Biol. Chem. 263:3790–3794.
Piacentini, M., Davies, P. J. A., and Fesus, L. (1994). Tissue transglutaminase in cells undergoing apoptosis. In Tomei, L. D., and Cope, F. O. (eds.), Apoptosis II: The Molecular Basis of Apoptosis in Disease, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 143–163.
Piredda, L., Amendola, A., Colizzi, V., Davies, P. J. A., Farrace, M. G., Fraziano, M., Gentile, V., Uray, I., Piacentini, M., and Fesus, L. (1997). Lack of “tissue” transglutaminase protein cross-linking leads to leakage of macromolecules from dying cells: relationship to development of autoimmunity in MRL lpr/lpr mice. Cell Death Differ. 4:463–472.
Raff, M. C., Barbes, B. A., Burne, J. F., Coles, H. S., Ishizaki, Y., and Jacobson, D. (1993). Programmed cell death and the control of cell survival: Lessons from the nervous system. Science 262:695–700.
Rasmussen, L. K., Sorensen, E. S., Petersen, T. E., Gliemann, J., and Jensen, P. H. (1994). Identification of glutamine and lysine residues in Alzheimer amyloid βA4 peptide responsible for transglutaminase-catalyzed homopolimerization and cross-linking to α 2M receptor. FEBS Lett. 338:161–166.
Roy, M., Mahadevan, M. S., McLean, M., Shutler, G., Yaraghi, Z., Farahani, R., Baird, S., Bresner-Johnston, A., Lefebvre, C., Kang, X., Salih, M., Aubry, H., Tamai, K., Guan, X., Ioannou, P., Crawford, T. O., de Jong, P. J., Surh, L., Ikeda, J.-E., Korneluk, R. G., and MacKenzie, A. (1995). The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell 80:167–178.
Schwartz, L. M., Smith, S. W., Jones, M. E. E., and Osborne, B. A. (1993). Do all programmed cell death occur via apoptosis? Proc. Natl. Acad. Sci. USA 90:980–984.
Sei, Y., Von Lubitz, K. J., Basile, A. I., Borner, M. M., Lin, R. L., Skolnick, P., and Fossom, L. H. (1994). Internucleosomal DNA fragmentation in gerbil hippocampus following forebrain ischemia. Neurosci. Lett. 171:179–182.
Selkoe, D. J., Abraham, C., and Ihara, Y. (1982). Brain transglutaminase: in vitro crosslinking of human neurofilament proteins into insoluble polymers. Proc. Natl. Acad. Sci. USA 79:6070–6074.
Sendtner, M., Holtmann, B., Kolbeck, R., Thoenen, H., and Barde, Y. A. (1992). Brain-derived neurotropic factor prevents the death of motoneurons in newborn rats after nerve section. Nature 360:757–759.
Steller, H. (1995). Mechanisms and genes of cellular Suicide. Science 267:1445–1448.
Thomazy, V., and Fesus, L. (1989). Differential expression of tissue transglutaminase in human cells. Cell Tissue Res. 255:215–224.
Villa, P., Miehe, M., Sensenbrenner, M., and Pettmann, B. (1994). Synthesis of specific proteins in trophic factor-deprived neurons undergoing apoptosis. J. Neurochem. 62:1468–1475.
Wyllie, A. H., Kerr, J. F. R., and Currie, A. R. (1980). Cell death: the significance of apoptosis. Int. Rev. Cytol. 68:251–306.
Yan, Q., Elliot, J., and Snider, W. D. (1992). Brain-derived neurotropic factor rescues spinal motor neurons from axotomy-induced cell death. Nature 360:753–755.
Zatloukal, K., Fesus, L., Denk, H., Tarcsa, E., Spurej, G., and Böck, G. (1992). High amount of ɛ(γ-glutamyl)lysine cross-links in Mallory bodies. Lab. Invest. 66:774–777.
Zhong, L.-T., Sarafian, T., Kane, D. J., Charles, A. C., Mah, S. P., Edwards, R. H., and Bredesen, D. E. (1993). Bcl-2 inhibits death of central neural cells induced by multiple agents. Proc. Natl. Acad. Sci. USA 90:4533–4537.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fesus, L. Transglutaminase-Catalyzed Protein Cross-Linking in the Molecular Program of Apoptosis and Its Relationship to Neuronal Processes. Cell Mol Neurobiol 18, 683–694 (1998). https://doi.org/10.1023/A:1020638020024
Issue Date:
DOI: https://doi.org/10.1023/A:1020638020024