Abstract
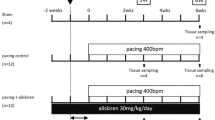
Rapid atrial pacing produces atrial systolic and diastolic failure characterized by absent atrial booster pump function, increased atrial chamber stiffness, enhanced atrial conduit function, and atrial enlargement. However, the processes underlying these abnormalities are poorly understood. Therefore, we examined left atrial myocardium from dogs with rapid pacing-induced atrial failure (400 bpm for 6 weeks) and from control dogs. Western blotting was used to determine the levels of proteins involved in calcium homeostasis (SERCA 2A, phospholamban, Na+-Ca2+ exchanger). Matrix metalloproteinase (MMP) activity was measured using gelatin and casein zymography, and levels of tissue inhibitor of metalloproteinase-4 (TIMP-4) and the TIMP-4 complexed with MMPs were measured with Western blot analysis. There were no differences in SERCA 2A or Na+-Ca2+ exchanger protein levels, but phospholamban level was significantly decreased in atrial samples from rapidly paced dogs (51.2 ± 7.8 vs. 77.0 ± 10.0, p < 0.01). The activity of MMP-9 was selectively and significantly increased by ∼ 50%, and the level of complexed TIMP-4 protein was significantly decreased by ∼ 50% in samples from dogs with atrial failure. Thus, rapid pacing-induced atrial failure is associated with differential changes in MMP activity, an unchanged number of calcium pumps, and compensatory changes in the level of phospholamban.
Similar content being viewed by others
References
Prystowsky E: Perspectives and controversies in atrial fibrillation. Am J Cardiol 82: 3I–6I, 1998
Hohnloser S, Li Y: Drug treatment of atrial fibrillation: What have we learned? Curr Opin Cardiol 12: 24–32, 1997
Jung F, DiMarco J: Treatment strategies for atrial fibrillation. Am J Med 104: 272–286, 1998
Sanfilippo A, Abascal V, Sheehan M, Oertel L, Harrigan P, Hughes R, Weyman A: Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation 82: 792–797, 1990
Sun H, Gaspo R, Leblanc N, Nattel S: Cellular mechanisms of atrial contractile dysfunction caused by sustained atrial tachycardia. Circulation 98: 719–727, 1998
Nattel S: Atrial electrophysiological remodeling caused by rapid atrial activation: underlying mechanisms and clinical relevance to atrial fibrillation. Cardiovasc Res 42: 298–308, 1999
Leistad E, Aksnes G, Verburg E, Christensen G: Atrial contractile dysfunction after short-term atrial fibrillation is reduced by verapamil but increased by BAY K8644. Circulation 93: 1747–1754, 1996
Gunja-Smith Z, Morales A, Romanelli R, Woessner J: Remodeling of human myocardial collagen in idiopathicdilated cardiomyopathy: role of metalloproteinases and pyridinoline cross-links. Am J Pathol 148: 1639–1648, 1996
Thomas C, Coker B, Zellner J, Handy J, Crumbley III AJ, Spinale F: Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation 97: 1708–1715, 1998
Spinale F, Coker ML, Thomas C, Walker J, Mukherjee R, Hebbar L: Time-dependent changes in matrix metalloproteinase activity and expression during the progression of congestive heart failure: Relation to ventricular and myocyte function. Circ Res 82: 482–495, 1998
Spinale F, Coker M, Krombach S, Mukherjee R, Hallak H, Houck W, Clair M, Kribbs S, Johnson L, Peterson J, Zile MR: Matrix metalloproteinase inhibition during the development of congestive heart failure: Effects on left ventricular dimensions and function. Circ Res 85: 364–376, 1999
Coker M, Thomas C, Clair M, Hendrick J, Krombach R, Galis Z, Spinale F: Myocardial matrix metalloproteinase activity and abundance with congestive heart failure. Am J Physiol 274: H1516–H1523, 1998
Weber K, Pick R, Silver M, Moe G, Janicki J, Zucker I, Armstrong P: Fibrillar collagen and remodeling of dilated canine left ventricle. Circulation 82: 1387–1401, 1990
Cleutjens J: The role of matrix metalloproteinases in heart disease. Cardiovasc Res 32: 816–821, 1996
Kohn EC, Jacobs W, Kim YS, Alessandro R, Stetler-Stevenson WG, Liotta LA: Calcium influx modulates expression of matrix metalloproteinase-2 (72-kDa type IV collagenase, gelatinase A). J Biol Chem 269: 21505–21511, 1994
Tyagi SC, Kumar S, Katwa L: Differential regulation of extracellular matrix metalloproteinase and tissue inhibitor by heparin and cholesterol in fibroblast cells. J Mol Cell Cardiol 29: 391–404, 1997
Hoit BD, Shao Y, Gabel M: Left atrial systolic and diastolic function accompanying chronic rapid pacing-induced atrial failure. Am J Physiol 275: H183–H189, 1998
Kadambi VJ, Ponniah S, Harrer JM, Hoit BD, Dorn GW II, Walsh RA, Kranias EG: Cardiac-specific overexpression of phospholamban alters calcium kinetics and resultant cardiomyocyte mechanics in transgenic mice. J Clin Invest 97: 533–539, 1996
Takeishi Y, Bhagwat A, Ball NA, Kirkpatrick DL, Periasamy M, Walsh RA: Effect of angiotensin-converting enzyme inhibition on protein kinase C and SR proteins in heart failure. Am J Physiol 276: H53–H62, 1999
Tyagi S, Kumar S, Haas S, Reddy H, Voelker D, Hayden M, Demmy T, Schmaltz R, Curtis J: Post-transcriptional regulation of extracellular matrix metalloproteinase in human heart end-stage failure secondary to ischemic cardiomyopathy. J Mol Cell Cardiol 28: 1415–1428, 1996
Tyagi S, Matsubara L, Weber K: Direct extraction and estimation of collagenase(s) activity by zymography in microquantities of rat myocardium and uterus. Clin Biochem 26: 191–198, 1993
Tyagi S, Ratajska A, Weber K: Myocardial matrix metalloproteinase(s): Localization and activation. Mol Cell Biochem 126: 49–59, 1993
Tyagi SC, Campbell SE, Reddy HK, Tjahja E, Voelker DJ: Matrix metalloproteinase activity expression in infarcted, noninfarcted and dilated cardiomyopathic human hearts. Mol Cell Biochem 155: 13-21, 1996
Hoit BD, Shao Y, Gabel M: Left atrial systolic and diastolic function accompanying chronic rapid pacing-induced atrial failure. Am J Physiol 275: H183–H189, 1998
Tyagi S, Kumar S, Voelker D, Reddy H, Janicki J, Curtis J: Differential gene expression of extracellular matrix components in dilated cardiomyopathy. J Cell Biochem 63: 185–198, 1996
Li YY, Feldman AM, Sun Y, McTiernan CF: Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation 98: 1728–1734, 1998
Li H, Simon H, Bocan T, Peterson J: MMP/TIMP expression in spontaneously hypertensive heart failure rats: The effect of ACE-and MMPinhibition. Cardiovasc Res 46: 298–306, 2000
Greene J, Wang M, Liu YE, Raymond LA, Rosen C, Shi YE: Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem 271: 30375–30380, 1996
Bigg HF, Shi YE, Liu YE, Steffensen B, Overall CM: Specific, high affinity binding of tissue inhibitor of metalloproteinases-4 (TIMP-4) to the COOH-terminal hemopexin-like domain of human gelatinase A. TIMP-4 binds progelatinase A and the COOH-terminal domain in a similar manner to TIMP-2. J Biol Chem 272: 15496-15500, 1997
Tummalapalli CM, Heath BJ, Tyagi SC: Tissue inhibitor of metalloproteinase-4 instigates apoptosis in transformed cardiac fibroblasts. J Cell Biochem 80: 512–521, 2001
Sensaki H, Paolocci N, Gluzband Y, Lindsey M, Janicki J, Crow M, Kass D: B-blockade prevents sustained metalloproteinase activation and diastolic stiffening induced by angiotensin II combined with evolving cardiac dysfunction. Circ Res 86: 807–815, 2000
Peterson J, Hallik H, Johnson L, Li H, O'Brien P, Sliskovic D, Bocan T, ML C, Etoh T, Spinale G: Matrix metalloproteinase inhibition attenuates left ventricular remodeling and dysfunction in a rat model of progressive heart failure. Circulation 103: 2303–2309, 2001
Cleutjens J, Kandala J, Guarda E, Guntaka R, Weber K: Regulation of collagen degradation in the rat myocardium after infarction. J Mol Cell Cardiol 27: 1281–1292, 1995
Daoud EG, Knight BP, Weiss R, Bahu M, Paladino W, Goyal R, Man KC, Strickberger SA, Morady F: Effect of verapamil and procainamide on atrial fibrillation-induced electrical remodeling in humans. Circulation 96: 1542–1550, 1997
Goette A, Honeycutt C, Langberg J: Electrical remodeling in atrial fibrillation: Time course and mechanisms. Circulation 94: 2968–2974, 1996
Tieleman R, DeLange C, VanGelder I, de Kam P, Grandjean J, Bel K, Wijffels M, Allessie M, Crijns H: Varapamil reduces tachycardia-induced electrical remodeling of the atria. Circulation 95: 1945–1953, 1997
Lai LP, Su MJ, Lin JL, Lin FY, Tsai CH, Chen YS, Huang SK, Tseng YZ, Lien WP: Down-regulation of L-type calcium channel and sarcoplasmic reticular Ca(2+)-ATPase mRNA in human atrial fibrillation without significant change in the mRNA of ryanodine receptor, calsequestrin and phospholamban: An insight into the mechanism of atrial electrical remodeling. J Am Coll Cardiol 33:1231–1237, 1999
Ohkusa T, Ueyama T, Yamada J, Yano M, Fujimura Y, Esato K, Matsuzaki M: Alterations in cardiac sarcoplasmic reticulum Ca2+ regulatory proteins in the atrial tissue of patients with chronic atrial fibrillation. J Am Coll Cardiol 34: 255–263, 1999
Cohn J, Ferrari R, Sharpe N: Cardiac remodeling-concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol 35: 569–582, 2000
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hoit, B.D., Takeishi, Y., Cox, M.J. et al. Remodeling of the left atrium in pacing-induced atrial cardiomyopathy. Mol Cell Biochem 238, 145–150 (2002). https://doi.org/10.1023/A:1019988024077
Issue Date:
DOI: https://doi.org/10.1023/A:1019988024077