Abstract
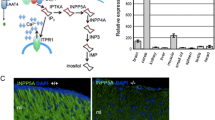
Point mutations and duplications of proteolipid protein (PLP) gene in mammals cause dysmyelination and oligodendrocyte cell death. The jimpy mouse, which has a lethal Plp point mutation, is the best characterized of the mutants; transgenic mice, which have additional copies of Plp gene, are less characterized. While oligodendrocyte death is a prominent feature in jimpy, the pathways leading to death have not been investigated in jimpy and Plp overexpressors. Using immunohistochemistry and immunobloting, we examined expression of cleaved caspase-3, Poly (ADP-ribose) polymerase (PARP), caspase-12, and mitochondrial apoptotic markers in spinal cord in jimpy males and Plp overexpressors. Compared to controls, cleaved caspase-3 is increased 10× in jimpy white matter spinal cord, and 3× in Plp overexpressor. In jimpy, the number of cleaved caspase-3 cells far exceeds the number of TUNEL+ cells. The majority of cleaved caspase-3+ cells were not TUNEL+ and these cells exhibited staining in perikarya and in processes. Only 30% of the cleaved caspase-3+ cells were TUNEL+ and exhibited both nuclear and perinuclear staining. This observation suggests that activation of caspase-3 begins earlier and overlaps for a period of time with DNA fragmentation. In both Plp mutants, quantitative immunobloting of PARP showed a 45% increase in total as well as cleaved form, indicating that oligodendrocytes die via apoptosis. Most interestingly, cleavage of caspase-12, a caspase associated with unfolded protein response, is dramatically increased in jimpy but not at all in Plp overexpressors. Mitochondrial markers cytochrome c and Bcl-XL are upregulated in both Plp mutants but levels of expression are different between mutants, suggesting that apoptosis in these two Plp mutants follows different pathways. In jimpy, mitochondrial apoptotic markers may play a role in amplifying the apoptotic signal. Our data shows for the first time, in vivo, that mutations in Plp gene increase oligodendrocyte death by activating the caspase cascade but the trigger to upregulate this cascade follows different pathways.
Similar content being viewed by others
References
Bansal, R., Stefansson, K. & Pfeiffer, S. E. (1992) Proligodendroblast antigen (POA), a developmental antigen expressed by A007/04-positive oligodendrocyte progenitors prior to the appearance of sulfatide and galactocerebroside. Journal of Neurochemistry 58, 2221–2229.
Bauer, J., Bradl, M., Klein, M., Leisser, M., Deckwerth, T. L., Wekerle, H. & Lassmann, H. (2002) Endoplasmic reticulum stress in PLP-overexpressing transgenic rats: Gray matter oligodendrocytes are more vulnerable than white matter oligodendrocytes. Journal of Neuropathology and Experimental Neurology 61, 12–22.
Beesley, J. S., Lavy, L., Eraydin, N. B., Siman, R. & Grinspan, J. B. (2001) Caspase-3 activation in oligodendrocytes fromthe myelin-deficient rat. Journal of Neuroscience Research 64, 371–379.
Bongarzone, E. R., Jacobs, E., Schonmann, V., Kampf, K., Campagnoni, C. W. & Campagnoni, A. T. (2001) Differential sensitivity in the survival of oligodendrocyte cell lines to overexpression of myelin proteolipid protein gene products. Journal of Neuroscience Research 65, 485–492.
Boucher, S. E. M., Cypher, M. A., Carlock, L. R. & Skoff, R. P. (2002) Proteolipid protein gene modulates viability and phenotype of neurons. Journal of Neuroscience 22, 1772–1783.
Bradl, M., Bauer, J., Inomata, T., Zielasek, J., Nave, K.-A., Toyka, K., Lassmann, H. & Wekerle, H. (1999) Transgenic Lewis rats overexpressing the proteolipid protein gene: Myelin degeneration and its effect on T cell-mediated experimental autoimmune encephalomyelitis. Acta Neuropathologica 97, 595–606.
Bredesen, D. E. (2000) Apoptosis: Overview and signal transduction pathways. Journal of Neurotrauma 17, 801–810.
Cardone, M. H., Roy, N., Stennicke, H. R., Salvessen, H. R., Franke, T. F., Stanbridge, E., Frisch, S. & Reed, J. C. (1998) Regulation of cell death protease caspase-9 by phosphorylation. Science 282, 1318–1321.
Cookson, M. R., Ince, P. G., Usher, P. A. & Shaw, P. J. (1999) Poly (ADP-ribose) polymerase is found in both the nucleus and cytoplasm of human CNS neurons. Brain Research 834, 182–185.
Denecker, G., Vercammen, D., Declercq, W. & Vandenabeele, P. (2001) Apoptoticandnecrotic cell death induced by death domain receptors. Cellular and Molecular Life Sciences 58, 356–370.
Feutz, A. C., Pham-Dinh, D., Allinquant, B., Miehe, M. & Ghandour, M. S. (2001) An immortalized jimpy oligodendrocyte cell line: Defects in cell cycle and cAMP pathway. Glia 34, 241–252.
Flores, A. I., Mallon, B. S., Matsui, T., Ogawa, W., Rosenzweig, A., Okamoto, T. & MacKlin, W. B. (2000) Akt-mediated survival of oligodendrocytes induced by neuregulins. Journal of Neuroscience 20, 7622–7630.
Fukuzono, S., Takeshita, T., Sakamoto, T., Hisada, A., Shimizu, N. & Mikoshiba, K. (1998) Overproduction and immuno-affinity purification of myelin proteolipid protein (PLP), an inositol hexakisphosphate-binding protein, in a baculovirus expression system. Biochemical and Biophysical Research Communications 249, 66–72.
Gardiner, L. & Hickson, I. D. (2002) Mechanisms of DNA damage and repair. Celltransmissions (Sigma-RBI) 18, 3–10.
Ghandour, M. S. & Skoff, R. P. (1988) Expression of galactocerebroside in developing normal and jimpy oligodendrocytes in situ. Journal of Neurocytology 17, 485–498.
Gow, A., Friedrich, V. L. & Lazzarini, R. A. (1994) Many naturally occurring mutations of myelin proteolipid protein impair its intracellular transport. Journal of Neuroscience Research 37, 574–583.
Gow, A., Southwood, C. M. & Lazzarini, R. A. (1998) Disrupted proteolipid protein trafficking results in oligodendrocyte apoptosis in an animal model of Pelizaeus-Merzbacher disease. Journal of Cell Biology 140, 925–934.
Grinspan, J. B., Coulalaglou, M., Beesley, J. S., Carpio, D. F. & Scherer, S. S. (1998) Maturationdependent apoptotic cell death of oligodendrocytes in myelin-deficient rats. Journal of Neuroscience Research 54, 623–634.
Gu, C., Casaccia-Bonnefil, P., Srinivasan, A. & Chao, M. V. (1999) Oligodendrocyte apoptosis mediated by caspase activation. Journal of Neuroscience 19, 3043–3049.
Hacki, J., Egger, L., Monney, L., Conus, S., Rosse, T., Fellay, I. & Borner, C. (2000) Apoptotic crosstalk between the endoplasmic reticulum and mitochondria controlled by Bcl-2. Oncogene 19, 2286–2295.
Hisahara, S., Yuan, J., Momoi, T., Okano, H. & Miura, M. (2001) Caspase-11 mediates oligodendrocyte cell death and pathogenesis of autoimmune-mediated demyelination. Journal of Experimental Medicine 193, 111–122.
Hudson, L. D. & Nadon, N. L. (1992) Amino acid substitution in proteolipid protein that cause dysmyelination. In Myelin: Biology and Chemistry (edited by Martenson, R. E.) pp. 677–702. Boca Raton: CRC Press.
Imaizumi, K., Miyoshi, K., Katayama, T., Yoneda, T., Taniguchi, M., Kudo, T. & Tohyama, M. (2001) The unfolded protein response and Alzheimer's disease. Biochimica et Biophysica Acta 1536, 85–96.
Kagawa, T., Ikenaka, K., Inoue, Y., Kuriyama, S., Tsujii, T., Nakao, J., Nakajima, K., Aruga, J., Okano, H. & Mikoshiba, K. (1994) Glial cell degeneration and hypomyelination caused by overexpression of myelin proteolipid protein gene. Neuron 13, 427–442.
Kaufmann, S. H., Desnoyers, S., Ottaviano, Y., Davidson, N. E. & Poirier, G. G. (1993) Specific proteolytic cleavage of poly (ADP-ribose) polymerase: An early marker of chemotherapy-induced apoptosis. Cancer Research 53, 3976–3985.
Kitagawa, K., Sinoway, M. P., Yang, C., Gould, R. M. & Colman, D. R. (1993) A proteolipid protein gene family: Expression in sharks and rays and possible evolution from an ancestral gene encoding a poreforming polypeptide. Neuron 11, 433–448.
Klugmann, M., Schwab, M., PÜhlhofer, A., Schneider, A., Zimmermann, F., Griffiths, I. R. & Nave, K.-A. (1997) Assembly of CNS myelin in the absence of proteolipid protein. Neuron 18, 69–70.
Knapp, P. E. (1996) Proteolipid protein: Is it more than just a structural component of myelin? Developmental Neuroscience 18, 297–308.
Knapp, P. E., Bartlett, W. P., Williams, L. A., Yamada, M., Ikenaka, K. & Skoff, R. P. (1999a) Programmed cell death without DNA fragmentation in the jimpy mouse: Secreted factors can enhance survival. Cell Death and Differentiation 6, 136–145.
Knapp, P. E., Dutta, S. & Skoff, R. P. (1990) Differences in levels of neuroglia cell death in jimpy male mice and carrier females. Developmental Neuroscience 12, 145–152.
Knapp, P. E., Ismaili, S., Hauser, K. F. & Ghandour, M. S. (1999b) Abnormal Ca2+ regulation in oligodendrocytes from the dysmyelinating jimpy mouse. Brain Research 847, 332–337.
Knapp, P. E., Skoff, R. P. & Redstone, D. W. (1986) Oligodendroglial cell death in jimpy mice: An explanation for the myelin deficit. Journal of Neuroscience 6, 2813–2822.
Lamarre, D., Talbot, B., de Murcia, G., Laplante, C., Leduc, Y., Mazen, A. & Poirier, G. G. (1988) Structural and functional analysis of poly (ADP ribose) polymerase: An immunological study. Biochimica et Biophysica Acta 950, 147–160.
Lazebnik, Y. A., Kaufmann, S. H., Desnoyers, S., Poirier, G. G. & Earnshaw, W. C. (1994) Cleavage of poly(ADP-ribose) polymerase by aproteinase with properties like ICE. Nature 371, 346–347.
Li, P.-A., He, Q.-P., Ouyang, Y.-B., Liu, C.-L., Hu, B.-R. & SIESJÖ, B. K. (2001) Early release of cytochrome c and activation of caspas-3 in hyperglycemic rats subjected to transient forebrain ischemia. Brain Research 896, 69–76.
Lipsitz, D., Goetz, B. D. & Duncan, I. D. (1998) Apoptotic glial cell death and kinetics in the spinal cord of the myelin-deficient rat. Journal of Neuroscience Research 51, 497–507.
Moldrich, R. X., Beart, P. M., Pascoe, C. J. & Cheung, N. S. (2000) Low-affinity kainate receptor agonists induce insult-dependent apoptosis and necrosis in cultured murine cortical neurons. Journal of Neuroscience Research 59, 788–796.
Nakagawa, T. & Yuan, J. (2000) Cross-talk between two cysteine protease families: Activation of caspase-12 by calpain in apoptosis. Journal of Cell Biology 150, 887–894.
Nave, K.-A., Bloom, F. E. & Milner, R. J. (1987) A single nucleotide difference in the gene for myelin proteolipid protein defines the jimpy mutation in mouse. Journal of Neurochemistry 49, 1873–1877.
Pieper, A. A., Verma, A., Zhang, J. & Snyder, S. H. (1999) Poly (ADP-ribose) polymerase, nitric oxide and cell death. Trends in Pharmacological Science 20, 171–181.
Rao, R. V., Hermel, E., Castro-Obregon, S., del Rio, G., Ellerby, L. M., Ellerby, H. M. & Bredesen, D. E. (2001) Coupling endoplasmic reticulum stress to the cell death program. Journal of Biological Chemistry 276, 33869–33874.
Readhead, C., Schneider, A., Griffiths, I. R. & Nave, K.-A. (1994) Premature arrest of myelin formation in transgenic mice with increased proteolipid protein gene dosage. Neuron 12, 583–595.
Roussel, G., Neskovic, N. M., Trifilieff, E., Artault, J.-C. & Nussbaum, J.-L. (1987) Arrest of proteolipid transport through the Golgi apparatus in jimpy brain. Journal of Neurocytology 16, 195–204.
Sakahira, H., Enari, M., Ohsawa, Y., Uchiyama, Y. & Nagata, S. (1999) Apoptotic nuclear morphological change without DNA fragmentation. Current Biology 9, 543–546.
Scurlock, B. & Dawson, G. (1999) Differential responses of oligodendrocytes to tumor necrosis factor and other pro-apoptotic agents: Role of ceramide in apoptosis. Journal of Neuroscience Research 55, 514–522.
Shibata, M., Hisahara, S., Hara, H., Yamawaki, T., Fukuuchi, Y., Yuan, J., Okano, H. & Miura, M. (2000) Caspases determine the vulnerability of oligodendrocytes in the ischemic brain. Journal of Clinical Investigation 106, 643–653.
Simons, M., KrÄmer, E.-A., Macchi, P., Rathkehartlieb, S., Trotter, J., Nave, K.-A. & Schulz, J. (2002) Overexpression of the myelin proteolipid protein leads to accumulation of cholesterol and proteolipid protein in endosomes/lysosomes: Implications for Pelizaeus-Merzbacher disease. Journal of Cell Biology 157, 327–336.
Skoff, R. P. (1995) Programmed cell death in the dysmyelinating mutants. Brain Pathology 5, 283–288.
Skoff, R. P. (2002) TUNEL (TdT-mediated dUTP nick end labeling). In Wiley Encyclopedia of Molecular Medicine (edited by Creighton, T. E.) pp. 3273–3277.New York: John Wiley & Sons.
Skoff, R. P., Ghandour, M. S. & Knapp, P. E. (1994) Postmitotic oligodendrocytes generated during postnatal cerebral development are derived from proliferation of immature oligodendrocytes. Glia 12, 12–23.
Southwood, C. & Gow, A. (2001) Molecular pathways of oligodendrocyte apoptosis revealed by mutations in the proteolipid protein gene. Microscopy Research and Technique 52, 700–708.
Springer, J. E., Azbill, R. D. & Knapp, P. E. (1999) Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nature Medicine 5, 943–946.
Thomson, C. E., Montague, P., Jung, M., Nave, K.-A. & Griffiths, I. R. (1997) Phenotypic severity of murine Plp mutants reflects in vivo and in vitro variations in transport of PLP isoproteins. Glia 20, 322–332.
Thornberry, N. A. & Lazebnik, Y. (1998) Caspases: Enemies within. Science 281, 1312–1316.
Williams, W. C. & Gard, A. L. (1997) in vitro death of jimpy oligodendrocytes: Correlation with onset of DM-20/PLP expression and resistance to oligodendrogliotrophic factors. Journal of Neuroscience Research 50, 177– 189.
Yamaguchi, Y., Ikenaka, K., Niinobe, M., Yamada, H. & Mikoshiba, K. (1996) Myelin proteolipid protein (PLP), but not DM-20, is an inositol hexakisphosphate-binding protein. Journal of Biological Chemistry 271, 27838–27846.
Yang, X. & Skoff, R. P. (1997) Proteolipid protein regulates the survival and differentiation of oligodendrocytes. Journal of Neuroscience 17, 2056–2070.
Zhu, C., Wang, X., Hagberg, H. & Blomgren, K. (2000) Correlation between caspase-3 activation and three different markers of DNA damage in neonatal cerebral hypoxia-ischemia. Journal of Neurochemistry 75, 819–829.
Rights and permissions
About this article
Cite this article
Cerghet, M., Bessert, D.A., Nave, KA. et al. Differential expression of apoptotic markers in jimpy and in Plp overexpressors: evidence for different apoptotic pathways. J Neurocytol 30, 841–855 (2001). https://doi.org/10.1023/A:1019697506757
Issue Date:
DOI: https://doi.org/10.1023/A:1019697506757