Abstract
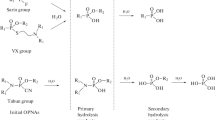
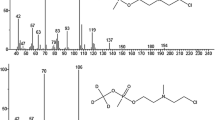
The main goal of chemical analytical monitoring in the destruction of chemical weapons is to identify highly toxic compounds of similar structures in long homologues series. The difficulties with the identification are due to a wide range of possible structures of such compounds with similar mass-spectral patterns. This necessitates the development of special approaches to the interpretation of analytical data. The paper presents the data of experimental studies of specific features of chromatographic–mass spectrometric behavior of 150 substances of the specified classes. The account of these data can improve the reliability of the identification.
Similar content being viewed by others
REFERENCES
The Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on Their Destruction GE 92–61926, Paris, 1993.
Molodtsov, S.G., Commun. Math. Chem. (MATCH), 1994, no. 30, p.213.
Kireev, A.F., Kholtsov, V.I., Rodyushkina, E.B., Rybalchenko, I.V., and Suvorkin, V.N., Proc. 1st Singapore Int. Symp. on Protection against Toxic Chemicals (1st SISPAT), Singapore, 1998.
Rybal'chenko, I.V., Tsekhmister, V.I., and Kireev, A.F., Zh. Ros. Khim. O-va im. D.I. Mendeleeva, 1994, vol.38, p. 13.
Yagupol'skii, L.M. and Ivanova, Z.M., Zh. Org. Khim., 1960, vol. 30, p.1284.
Tammelin, L.E., Acta Chem. Scand., 1957, vol. 11, p.-1738.
Fukuto, T.R. and Stafford, E.M., J. Am. Chem. Soc., 1957, vol. 79, p.6083.
HP 5890 Series II and HP 5890 Series II Plus Operating Manual. Edition 6, USA: Hewlett-Packard, 1993.
HP G1094A MS/IR ChemStation (DOS Series), IRD System Handbook, USA: Hewlett-Packard, 1993.
Jurs, P.C. and Izenhour, T.L., Chemical Applications of Pattern Recognition, New York: Wiley, 1975. Translated under the title Raspoznavanie obrazov v khimii, Moscow: Mir, 1977.
HP G1034C MS ChemStation User Guide, USA: Hewlett-Packard, 1993.
Raznikov, V.V. and Raznikova, M.O., Informatsionnoanaliticheskaya mass-spektrometriya (Informational Analytical Mass Spectrometry), Moscow: Nauka, 1991.
Spectrometric Identification of Organic Compounds, Silverstein, R.M., Bassler, G.C., and Morrill T.C., Eds., New York: Wiley, 1974. Translated under the title Spektrometricheskaya identifikatsiya organicheskikh soedinenii,Moscow: Mir, 1977.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kireev, A.F., Rybal'chenko, I.V., Savchuk, V.I. et al. Qualitative Chromatographic–Mass Spectrometric Analysis of Highly Toxic Alkyl Phosphonate and Alkyl Thiophosphonate Derivatives. Journal of Analytical Chemistry 57, 708–716 (2002). https://doi.org/10.1023/A:1016873825817
Issue Date:
DOI: https://doi.org/10.1023/A:1016873825817