Abstract
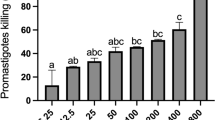
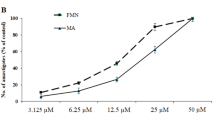
A galactomannan (GMPOLY) isolated from lichen Ramalina celastri was complexed with vanadyl ion (IV;VO) forming the complex GMPOLY-VO. Both GMPOLY and GMPOLY-VO diminished the superoxide anion production by macrophages triggered with PMA, the complex giving rise to this effect at concentrations 100 times lower than GMPOLY. Macrophages treated with GMPOLY enhanced the nitric oxide production (40%), this effect not being observed when interferon-γ ((IFN-γ) or IFN-γ plus lipopolysaccharide (LPS) were present. No effect on nitric oxide production was observed by treatment of macrophage with GMPOLY-VO. Both GMPOLY and GMPOLY-VO exhibited leishmanicidal effects on the amastigote form of Leishmania amazonesis, but only GMPOLY-VO inhibited the growth of promastigote form.
Similar content being viewed by others
References
Bohn JA, BeMiller JN: (1→3)-D-Glucans as biological response modifiers: A review of functional activity relationships. Carbohydr Polym 28: 3–14, 1995
Müller A, Rice PJ, Ensley HE, Coogan PS, Kalbfleisch JH, Kelley JL, Love EJ, Portera CA, Ha T, Browder IW, Williams DL: Receptor binding and internalization of a water-soluble (1→3)-β-D-Glucan biologic response modifier in two monocyte/macrophages cell lines. J Immunol 156: 3418–3425, 1996
Konopski Z, Smedsrod B, Seljelid R, Eskelund T: A novel immunomodulator soluble aminated β-1,3-D-glucan: Binding characteristics to mouse peritoneal macrophages. Biochem Biophys Acta 1221: 61–65, 1994
Kuliche WM, Lettau AI, Thielking H: Correlation between immunological activity, molar mass, and molecular structure of different (1→3)-β-D-glucans. Carbohydr Res 297: 135–143, 1997
Cohen MD, McManus TP, Yang Z, Qu Q, Schlesinger RB, Zelikoff J: Vanadium affects macrophages interferon-γ-binding and inducible responses. Toxicol Appl Pharmacol 138: 110–120, 1996
Tsuji A, Sakurai H: Vanadyl ion suppresses nitric oxide production from peritoneal macrophages of streptozotocin-induced diabetic mice. Biochem Biophys Res Commun 226: 506–511, 1996
Grabowski AM, Paulauski JD, Goldleski JJ: Mediating phosphorylation events in the vanadium-induced respiratory burst of alveolar macrophages. Toxicol Appl Pharmacol 156: 170–178, 1999
Demleitner S, Kraus J, Franz G: Synthesis and antitumour activity of derivatives of curdlan and lichenan branched at C-6. Carbohydr Res 226: 1239–246, 1992
Badway JA, Karnovski ML: Active oxygen species and the functions of phagocytic leukocytes. Ann Rev Biochem 49: 695, 1980
Berton G, Gordon S: Modulation of macrophages mannosyl; specific receptors by cultivation on immobilized zymosan. Effects on superoxide-anion release and phagocytosis. Immunology 49: 705–715, 1983
Ramamoorthy L, Kemp MC, Tizard IR: Acemannan, a β-(1→4)-acetylated mannan, induces nitric oxide production in macrophages cell line RAW 264.7. Mol Pharmacol 50: 878–884, 1996
Hashimoto T, Ohno N, Yadomae T: Subgroupping immunomodulating β-glucans by monitoring IFN-γ and NO syntheses. Drug Dev Res 42: 35–40, 1997
Cross GG, Jennings HJ, Whitefield DM, Penny CL, Zacharie B, Gagnon L: Immunostimulant oxidized β-glucan conjugates. Int Immunopharmacol 1: 539–550, 2001
Morinville A, May Singer D, Shaver A: From Vanadis to Atrops: Vanadium compounds as pharmacological tools in cell death signalling. Trends Pharmacol Sci 19: 452–460, 1998
Crans DC: Chemistry and insulin-like properties of vanadium (IV) and vanadium (V) compounds. J Inorg Biochem 80: 123–131, 2000
Yasui H, Takechi K, Sakurai H: Metallokinetic analysis of disposition of vanadyl complexes as insulin-mimetics in rats using BCM-ERS method. J Inorg Biochem 78: 185–196, 2000
Godwaser I, Gefel D, Gerhonov E, Fridkin M, Shechter Y: Insulin-like effects of vanadium: Basic and clinical implications. J Inorg Biochem 80: 21–25, 2000
Cortizo AM, Bruzzone L, Molinuevo S, Etcheverry SB: A possible role of oxidative stress in the vanadium-induced cytotoxicity in the MC3T3E1 osteoblast and UMR106 osteosarcoma cell lines. Toxicol 147: 89–99, 2000
Sreedhara A, Nobuyuki S, Patwardham A, Rao CP: One electron reduction of vanadate (V) by low-molecular-weight biocomponents like saccharides and ascorbic acid: Effect of oxovanadium (IV) complexes on pUC18 DNA and on lipid peroxidation in isolated rat hepatocytes. Biochem Biophys Res Commun 224: 115–120, 1996
Cortizo AM, Caporossi M, Lettieri G, Etcheverry SB, Vanadate induced nitric oxide production role in osteoblast growth and differentiation. Eur J Pharmacol 400: 279–285, 2000
Matte C, Marquis J-F, Blanchette J, Gros P, Faure R, Posner BI, Olivier M: Peroxovanadium-mediated protection against murine leishmaniasis: Role of the modulation of nitric oxide. Eur J Immunol 30: 2555–22264, 2000
Tenth Program Report of the UNDP/World/Bank/WHO Special Program for Research and training in Tropical Diseases (TDR), 1991, 79
Roberts WL, Hariprashad J, Rainey PM, Murray WH: Pentavalent antimony conjugate therapy of experimental visceral leishaminiasis. Am J Trop Med Hyg 55: 444–446, 1996
Cantos G, Barbieri C, Iacomini M, Gorin PAJ, Travassos LR: Synthesis of antimony complexes of yeast mannan and mannan derivatives and their effect on Leishmania-infected macrophages. Biochem J 289: 155–160, 1993
Etcheverry SB, Williams PAM, Baron EJ: Synthesis and characterization of oxovanadium (IV) complexes with saccharides. Carbohydr Res 320: 131–138, 1997
Verchére JF, Chapelle S, Xin F, Crans DC: Metal-carbohydrate complexes in solution. Prog Inorg Chem 47: 837–945, 1998
Hudson FTG: In: Toxicological and Biological Significance. Elsevier, New York, 1964, 140
Miceno AM, Gorin PAJ, Iacomini M: Galactomannan and isolichenan components of the carbohydrate-rich lichen Ramalina eckloni (Spreng.) May and Flot. Agric Biol Chem 55: 1391–1392, 1991
Mercê ALR, Spir IHZ, Salmón MJ O, Giannoni RA, Mangrich AS: Model compounds of humic acid and oxovanadium cations. Potentiometric titration and EPR spectroscopy studies. J Braz Chem Soc 10: 463–468, 1999
Furman NH: In: Standard Methods of Chemical Analysis. Vol. I, The Elements. D. van Nostrand Company Inc., New York, 1962
Martell AE, Moteikatis RJ: In: The Determination and Use of Stability Constants. VCH, New York, 1992
Mercê ALR, Szpoganicz B, Dutra RC, Khan X, Thanh D, Bouet G: Potentiometric study of vitamin D3 complexes with cobalt (II), nickel (II) and copper (II) in water-ethanol medium. J Inorg Biochem 71: 87–91, 1998
Mercê ALR, Lombardi SC, Mangrich AS, Szpoganicz B, Reicher F, Sierakowski MR: Equilibrium studies of galactomannan of Cassia fastuosa and Leucaena leucocephala and Cu2+ using potentiometry and EPR spectroscopy. Carbohydr Polym 35: 13–20, 1998
Sasada M, Pabt MJ, Johnston RB Jr: Activation of mouse peritoneal macrophages by lipopolysaccharide alters the kinetic parameters of the superoxide-producing NADPH oxidase. J Biol Chem 258: 9631–9635, 1983
Reilly TP, Belleveue FH III, Worster PM, Svesson CK: Comparison of the in vitro cytotoxicity of hydroxilamine metabolites of sulfamethoxazole and dapsone. Biochem Pharmacol 55: 803–808, 1998
Johnston RB Jr, Godzik CA, Cohn ZA: Increase superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med 48: 115–127, 1978
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR: Analysis of nitrate, nitrite, and [15N nitrates] in biological fluids. Anal Biochem 126: 131–138, 1982
Olivier M, Romero-Gallo BJ, Matte C, Blanchette J, Posner BI, Tremblay MJ, Faure R: Modulation of interferon-γ-induced macrophages activation by phosphotyrosine phosphatase inhibition. J Biol Chem 273: 13944–13949, 1998
Lowry OH, Rosebrough NJ, Farr AC, Radal RJ: Protein measurement with the folin phenol reagent. J Biol Chem 193: 265–275, 1951
Bradford M: A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976
Baes CF, Mesmer RE: In: The Hydrolysis of Cations. Wiley, New York, 1976
Ciardelle F, Tsuchida E, Wöhrle D: In: Macromolecule-Metal Complexes. Spring, Germany, 1995
Sreedhara A, Srinivasa R, Rao CP: Transition metal-saccharide interactions: Synthesis and characterization of vanadyl saccharides. Carbohydr Res 264: 227–235, 1994
Cunha FQ, Assreuy J, Moncada S, Liew FY: Phagocytosis and induction of nitric oxide synthase in murine macrophages. Immunology 79: 408–411, 1993
Molyneux DH, Killick-Kendrick R: Morphology, ultrastructure and lifes cycles. In: W. Petters, R. Killick-Kendrick (eds). The Leishmaniases in Biology and Medicine: Biology and Epidemiology. Academic Press, London, 1987, pp 121–176
Mercê ALR, Fernandes E, Mangrich AS, Sierakowiski MR: Evaluation of the complexes of galactomannan for Leucaena Leucocephala and Co2+, Ni2+ and Zn2+. J Braz Chem Soc 11: 224–231, 2000
Malthlouthi M, Koening JL: Vibrational spectra of carbohydrates. Adv Carbohydr Chem Biochem 44: 7–89, 1986
Williams JD, Topley N, Alobaidi HM, Harber MJ: Activation of human polymorphonuclear leucocytes by particulate zymosan is related to both its major carbohydrate components: Glucan and mannan. Immunology 58: 117–125, 1986
Yamaguchi M, Oishi H, Araki S, Saeki S, Yamanae H, Okamura N, Ishibashi S: Respiratory burst and tyrosine phosphorylation by vanadate. Arch Biochem Biophys 323: 328–386, 1995
Jun PZ, Yuan C, Mei Z, Wan J: The effect of polysaccharide krestin on nitric oxide production in mouse peritoneal macrophages. Med Sci Res 27: 299–302, 1999
Deuk H-M, Sook LE, Kweon KY, Woo J, Hoon J, Há YK: Production of nitric oxide in raw 264.7 macrophages treated with ganoderan, the beta-glucan of Gonoderma lucidum. Korean J Mycol 26: 246–255, 1998
Kim S-W: Nitric oxide production ability and its formation mechanisms in macrophages TIB 71 cell line by polysaccharide extracted from Gonoderma lucidum. J Korean Soc Food Sci Nutrition 27: 333–337, 1998
Tsiapali E, Whaley S, Kalbfleisch J, Ensley HE, Browder WI, Williams DL: Glucans exhibit weak antioxidant activity, but stimulate macrophages free radical activity. Free Rad Biol Med 30: 393–402, 2001
Ding AH, Nathan CF, Stuehr DJ: Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. J Immunol 141: 2407–2412, 1988
Iyengar R, Stuehr DJ, Marletta MA: Macrophages synthesis of nitrite, nitrate and N-nitrosamines: Precursors and role of the respiratory burst. Proc Natl Acad Sci 84: 6369–6373, 1987
Murray HW: Susceptibility of Leishmania to oxygen intermediates and killing by normal macrophages. J Exp Med 153: 1302–1315, 1981
Buchmüller-Rouiller Y, Maüel J: Impairment of the oxidative metabolism of mouse peritoneal macrophages by intracellular Leishmania ssp. Infect Immun 55: 587–593, 1987
Buchmüller-Rouiller Y, Corradin SB, Smith J, Mauël J: Effect of increasing intravesicular pH on nitrite production and leishmanicidal activity of activated macrophages. Biochem J 301: 243–247, 1994
Rights and permissions
About this article
Cite this article
Noleto, G.R., Mercê, A.L.R., Iacomini, M. et al. Effects of a lichen galactomannan and its vanadyl (IV) complex on peritoneal macrophages and leishmanicidal activity. Mol Cell Biochem 233, 73–83 (2002). https://doi.org/10.1023/A:1015566312032
Issue Date:
DOI: https://doi.org/10.1023/A:1015566312032