Abstract
During insulin-dependent diabetes mellitus, islet invading immune cells destroy beta cells over a prolonged asymptomatic pre-diabetic period. Cytokines synthesised and secreted by specific immune cells within the islet infiltrate may be crucial effectors of beta cell destruction or protection during the disease. Interleukin-1β may be a key cytokine which may act in concert with other cytokines in initiating and/or promoting beta cell destruction. We have examined this hypothesis in NOD mice by assessing the intra-islet expression and co-localization of interleukin-1β at different time-points following cyclophosphamide administration. We have also tested the effects of long-term oral nicotinamide given to NOD mice in suppressing intra-islet expression of the cytokine in this accelerated model.
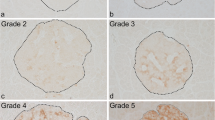
Cyclophosphamide was administered to day 95 female NOD mice. Pancreatic tissues were examined by dual-label confocal immunofluorescence microscopy for the expression and co-localization of interleukin-1β at days 0, 4, 7, 11 and at onset of diabetes (day 14). Diabetes developed in 7/11 mice 14 days after administration of cyclophosphamide while nicotinamide completely prevented the disease. At day 0, interleukin-β immunolabelling was observed in selective intra-islet macrophages, several somatostatin cells and in a few beta cells. However, at day 4, it was seen mostly in somatostatin and some beta cells. At day 7, an increasing number of interleukin-1β cells were observed within the islets and co-localized to several somatostatin cells, beta cells and macrophages. The mean number of intra-islet interleukin-1β cells reached a peak at day 11 and was significantly higher than at day 7 (p = 0.05) and at day 14 (onset of diabetes; p = 0.03). At day 11, interleukin-1β immunolabelling was also present in selective macrophages which co-expressed inducible nitric oxide synthase. At onset of diabetes, some macrophages, residual beta cells and somatostatin cells showed immunolabelling for the cytokine. Exposure of NOD mice to oral nicotinamide was associated with a considerably reduced expression of interleukin-1β cells within the islet at day 11 (p = 0.002). We conclude that cylophosphamide treatment enhances the expression of interleukin-1β in selective macrophages, somatostatin and beta cells during the course of the disease. Its expression reaches a maximum immediately prior to onset of diabetes. Interleukin-1β present in intra-islet macrophages, somatostatin and beta cells may influence its expression by autocrine and paracrine means. Interleukin-1β expression within islet macrophages may also up-regulate inducible nitric oxide synthase within the same macrophage or adjacent macrophage populations. These intra-islet molecular events may corroborate with other local cytotoxic processes leading to beta cell destruction. Oral nicotinamide may attenuate intra-islet expression of interleukin-1β and thus inducible nitric oxide synthase during prevention of Type 1 diabetes in this animal model. The expression of interleukin-1β in specific islet endocrine cell-types shown in this study requires furtherbreak investigation.
Similar content being viewed by others
References
Andersen HU, Jorgensen KH, Egeberg J, Mandrup-Poulsen T, Nerup J (1994) Nicotinamide prevents interleukin-1 effects on accumulated insulin release and nitric oxide production in rat islets of Langerhans. Diabetes 43: 770–777.
Andrade J, Conde M, Ramirez R et al. (1996) Protection from nicotinamide inhibition of interleukin-1β-induced RIN cell nitric oxide formation is associated with induction of MnSOD enzyme activity. Endocrinology 137: 4806–4810.
Arnush M, Heitmeier MR, Scarim AL et al.(1998a) IL-1 produced and released endogenously within human islets inhibits ? cell function. J Clin Invest 102: 516–526.
Arnush M, Scarim AL, Heitmeier MR et al. (1998b) Potential role of resident islet macrophage activation in the initiation of autoimmune diabetes. J Immunol 160: 2684–2691.
Bach JF (1994) Insulin-dependent diabetes mellitus as an autoimmune disease. Endocr Rev 15: 516–542.
Bendtzen K, Mandrup-Poulsen T, Nerup J, Nielsen JH, Dinarello CA (1986) Cytotoxicity of human pI interleukin-1 for pancreatic islets of Langerhans. Science 232: 1545–1547.
Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PGF, Gamble DR(1985) In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med 313: 353–360.
Cailleau C, Diu-Hercend A, Ruuth E, Westwood R, Carnaud C (1997) Treatment with neutralising antibodies specific for IL-1? prevents cyclophosphamide-induced diabetes in non-obese diabetic mice. Diabetes 46: 937–940.
Campbell IL, Cutri A, Wilson A, Harrison LC (1989) Evidence for IL-6 production by and effects on the pancreatic β-cell. J Immunol 143: 1188–1191.
Cetkovic-Cvrlje M, Eizirik DL (1994) TNF-α and IFN-γ potentiates the deleterious effects of IL-1β on mouse pancreatic islets mainly via generation of nitric oxide. Cytokine 6: 399–406.
Cetkovic-Cvrlje M, Sandler S, Eizirik DL (1993) Nicotinamide and dexamethasone inhibit interleukin-1-induced nitric oxide production by RINm5F cells without decreasing messenger ribonucleic acid expression for nitric oxide synthase. Endocrinology 133: 1739–1743.
Dinarello CA (1991) Interleukin-1 and interleukin-1 antagonism. Blood 77: 1627–1652.
Eisenbarth GS (1986) Type 1 diabetes mellitus. A chronic autoimmune disease. N Engl J Med 314: 1360–1368.
Eizirik DL, Cagliero E, Bjorklund A, Welsh N (1992) Interleukin-1β induces the expression of an isoform of nitric oxide synthase in insulin-producing cells, which is similar to that observed in activated macrophages. FEBS Lett 308: 249–252.
Eizirik DL, Sandler S, Welsh N, Cetkovic-Cvrlje M, Nieman A, Geller DA, Pipeleers DG, Berndtzen K, Hellerstrom C (1994) Cytokines suppress human islet function irrespective of their effects on nitric oxide generation. J Clin Invest 93: 1968–1974.
Elliott RB, Chase HP (1991) Prevention or delay of type 1 (insulin-dependent) diabetes mellitus in children using nicotinamide. Diabetologia 34: 362–365.
Foulis AK, Farquharson MA, Meager A (1987) Immunoreactive α-interferon in insulin-secreting β cells in type 1 diabetes mellitus. Lancet 2: 1423–1427.
Foulis AK, Liddle CN, Farquharson MA, Richmond JA, Weir RS (1986) The histopathology of the pancreas in type 1 (insulin-dependent) diabetes mellitus: a 25-year review of deaths in patients under 20 years of age in the United Kingdom. Diabetologia 29: 267–274.
Foulis AK, McGill M, Farquharson MA(1991) Insulitis in type 1 (insulindependent) diabetes mellitus in man-macrophages, lymphocytes, and interferon-γ containing cells. J Pathol 165: 97–103.
Fukuzawa M, Satoh J, Muto G, Nischimura S, Miyaguchi S, Qing XL, Toyota T (1997) Inhibitory effect of nicotinamide on in vitro and in vivo production of tumour necrosis factor-α. Immunol Lett 59: 7–11.
Hammonds P, Beggs M, Beresford G, Espinal J, Clarke J, Mertz RJ (1990) Insulin-secreting β-cells possess specific receptors for interleukin-1β. FEBS Lett 261: 97–100.
Hancock WW, Polanski M, Zhang J, Blogg N, Weiner HL (1995) Suppression of insulitis in non-obese diabetic (NOD) mice by oral insulin administration is associated with selective expression of interleukin-4 and-10, transforming growth factor-β, and prostaglandin-E. Am J Pathol 147: 1193–1199.
Hauschildt S, Scheipers P, Bessler WG (1991) Inhibitors of poly(ADPribose) polymerase suppress lipopolysaccharide-induced nitrite formation in macrophages. Biochem Biophys Res Commun 179: 865–871.
Hauschildt S, Scheipers P, Bessler WG, Mulsch A (1992) Induction of nitric oxide synthase in L929 cells by tumour necrosis factor-α is prevented by inhibitors of poly(ADP-ribose) polymerase. Biochem J 288: 255–260.
Honegger J, Spagnoli A, D'urso R, Navarra P, Tsagarakis S, Besser GM, Grossman AB (1991) Interleukin-1β modulates the acute release of growth hormone-releasing hormone and somatostatin from rat hypothalamus in vitro, whereas tumor necrosis factor and interleukin-6 have no effect. Endocrinology 129: 1275–1282.
Huang X, Yuan J, Goddard A et al. (1995) Interferon expression in the pancreases of patients with type I diabetes. Diabetes 44: 658–664.
Itoh N, Hanafusa T, Miyazaki A et al. (1993) Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion molecules in pancreas biopsy specimens from newly diagnosed insulin-dependent diabetes patients. J Clin Invest 92: 2313–2322.
Jacob CO, Aiso S, Mitchie SA, McDevitt HO, Acha-Orbea H (1990) Prevention of diabetes in non-obese diabetic mice by tumour necrosis factor (TNF). Similarities between TNF-α and interleukin. Proc Natl Acad Sci USA 87: 968–972.
Jafarian-Tehrani M, Amrani A, Homo-delarche F, Marquette C, Dardenne M, Haour F (1995) Localization and characterization of interleukin-1 receptors in the islets of Langerhans from control and non-obese diabetic mice. Endocrinology 136: 609–613.
Kay TWH, Campbell IL, Harrison LC (1991) Characterization of pancreatic T lymphocytes associated with beta cell destruction in the nonobese diabetic (NOD) mouse. J Autoimmun 4: 263–276.
Kolb H, Burkart V, Appels B, Hanenberg H, Kantwerk-Funke G, Kiesel U, Funda J, Schraermeyer U,Kolb-Bachofen V(1990) Essential contribution of macrophages to islet cell destruction in vivo and in vitro. J Autoimmun 3: 117–120.
Kretowski A, Mysliwiec J, Szelachowska M, Kinalski M, Kinalska I (2000) Nicotinamide inhibits enhanced in vitro production of interleukin-12 and tumour necrosis factor-α in peripheral whole blood of people at high risk of developing Type 1 diabetes and people with newly diagnosed Type 1 diabetes. Diabetes Res Clin Prac 47: 81–86.
Kwon G, Corbett JA, Rodi CP, Sullivan P, McDaniel ML (1995) Interleukin-1β-induced nitric oxide synthase expression by rat pancreatic β-cells: evidence for the involvement of nuclear factor βB in the signalling mechanism. Endocrinology 136: 4790–4795.
Lechan, RM, Toni R, Clark BD, Cannon JG, Shaw AR, Dinarello CA, Reichlin S (1990) Immunoreactive interleukin-1β localization in the rat forebrain. Brain Res 514: 135–140.
Loweth AC, Williams GT, James RFL, Scarpello JHB, Morgan NG (1998) Human islets of Langerhans express Fas ligand and undergo apotosis in response to interleukin-1β and Fas ligation. Diabetes 47: 727–732.
Mandrup-Poulsen T (1996) The role of interleukin-1 in the pathogenesis of IDDM. Diabetologia 39: 1005–1029.
Mandrup-Poulsen T, Bendtzen K, Nielsen JH, Bendixen G, Nerup J (1985) Cytokines cause functional and structural damage to isolated islets of Langerhans. Allergy 40: 424–429.
Mandrup-Poulsen T, Helqvist S, Molvig J, Wogensen LD, Nerup J (1989) Cytokines as immune effector molecules in autoimmune endocrine diseases with special reference to insulin-dependent diabetes mellitus. Autoimmunity 4: 191–218.
Mandrup-Poulsen T, Helqvist S, Wogensen LD, Molvig J, Pociot F, Johannsen J, Nerup J (1990) Cytokines and free radicals as effector molecules in the destruction of pancreatic beta cells. Current Topics Microbiol Immunol 164: 169–173.
Moriwaki M, Itoh N, Miyagawa J, Yamamoto K et al. (1999) Fas and Fas ligand expression in inflamed islets in pancreas sections of patients with recent-onset Type I diabetes mellitus. Diabetologia 42: 1332–1340.
Mossman TR, Coffman RL(1989) Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Ann Rev Immunol 7: 145–173.
Nicoletti F, Dimarco R, Barcellini W et al. (1994) Protection from experimental autoimmune diabetes in the non-obese diabetic mouse with soluble interleukin-1 receptor. Eur J Immunol 24: 1843–1847
Oliver FJ, Menissier-de Murcia J, Nacci C et al. (1999) Resistance to endotoxic shock as a consequence of defective NF-κB activation in poly (ADP-ribose) polymerase-1 deficient mice. EMBO J 18: 4446–4454.
Pilstrom B, Bjork L, Bohme J (1997) Monokine-producing cells predominate in the recruitment phase of NOD insulitis while cells producing Th1-type cytokines characterize the effector phase. J Autoimmun 10: 147–155.
Pociot F, Reimers JI, Andersen HU (1993) Nicotinamide: biological actions and therapeutic potential in diabetes prevention. Diabetologia 36: 574–576.
Pozzilli P (1998) Prevention of insulin-dependent diabetes mellitus. Diabetes/Metab Rev 14: 69–84.
Rabinovitch A (1994) Immunoregulatory and cytokine imbalances in the pathogenesis of IDDM: Therapeutic intervention by immunostimulation? Diabetes 43: 613–621.
Rabinovitch A (1998) An update on cytokines in the pathogenesis of insulin-dependent diabetes mellitus. Diabetes Metab Rev 14: 129–151.
Rabinovitch A, Suarez-Pinson WL, Strydnaka K, Schultz R, Lakey, Warnock GL et al. (1994) Human pancreatic islet β destruction by cytokines is independent of nitric oxide production. J Clin Endocrinol Metab 79: 1058–1062.
Rapport MJ, Jaramillo A, Zipris D et al. (1993) IL-4 reverses T-cell proliferative unresponsiveness and prevents the onset of diabetes in NOD mice. J Exp Med 178: 87–99.
Reddy S, Bibby NJ, Elliott RB (1990) Early nicotinamide treatment in the NOD mouse: effects on diabetes and insulitis suppression and autoantibody levels. Diabetes Res 15: 95–102.
Reddy S, Salari-Lak N, Sandler S (1995) Long-term effects of nicotinamide induced inhibition of poly(adenosine diphosphate-ribose) polymerase activity in rat pancreatic islets exposed to interleukin-1γ. Endocrinology 136: 1907–1912.
Reddy S, Wu D, Swinney C, Elliott RB (1995) Immunohistochemical analyses of pancreatic macrophages and CD4 and CD8 T cell subsets prior to and following diabetes in theNODmouse.Pancreas 11: 16–25.
Reddy S, Yip S, Karanam M, Poole CA, Ross JM (1999) An immunohistochemical study of macrophage influx and the co-localization of inducible nitric oxide synthase in the pancreas of non-obese (NOD) mice during disease acceleration with cyclophosphamide. Histochemical J 31: 303–314.
Reddy S, Karanam M, Krissansen G, Nitschke K, Neve J, Poole CA, Ross JM (2000a) Temporal relationship between immune cell influx and the expression of inducible nitric oxide synthase, interleukin-4 and interferon-γ in pancreatic islets of NOD mice following adoptive transfer of diabetic spleen cells. Histochemical J 32: 195–206.
Reddy S, Karanam M, Poole CA, Ross JM (2000b) Dual-label immunohistochemical study of interleukin-4-and interferon-γ-expressing cells within the pancreas of the NOD mouse during disease acceleration with cyclophosphamide. Autoimmunity 32: 181–192.
Reddy S, Karanam M, Robinson E (2001) Prevention of cyclophosphamide-induced accelerated diabetes in the NOD mouse by nicotinamide or a soy protein-based infant formula. Int J Exptl Diabetes Res 1: 299–313.
Sandler S, Andersson A, Hellerstrom C (1987) Inhibitory effects of interleukin-1 on insulin secretion, insulin biosynthesis, and metabolism of isolated rat pancreatic rat islets. Endocrinology 121: 1424–1431.
Somoza N, Vargas F, Roura-Mir C et al. (1994) Pancreas in recent onset insulin-dependent diabetes mellitus. Changes in HLA, adhesion molecules and autoantigens, restricted T cell receptor Vγ usage, and cytokine profile. J Immunol 153: 1360–1377.
Southern C, Schulster D, Green IC (1990) Inhibition of insulin secretion by interleukin-1β and tumour necrosis factor-α via an L-argininedependent nitric oxide generating mechanism. FEBS Lett 276: 42–44.
Stassi G, De Maria R, Trucco G, Rudert W, Testi R, Galluzzo A, Giordano C, Trucco M (1997) Nitric oxide primes pancreatic β cells for Fas-mediated destruction in insulin-dependent diabetes mellitus. J Exp Med 186: 1193–1200.
Stassi G, Todaro M, Richiusa P et al. (1995) Expression of apoptosis-inducing CD95 (Fas/Apo-1) on human β-cells sorted by flow-cytometry and cultured in vitro. Transplant Proc 27: 3271–3275.
Suarez-Pinzon W, Sorensen O, Bleackley RC, Elliott JF, Rajotte RV, Rabinovitch A (1999) β-cell destruction in NOD mice correlates with Fas(CD95) expression on β-cells and proinflammatory cytokine expression in islets. Diabetes 48: 21–28.
Szabo C, Virag L, Cuzzocrea S et al. (1998) Protection against peroxynitrite-induced fibroblast injury and arthritis development by inhibition of poly(ADP-ribose) synthatase. Proc Natl Acad Sci USA 95: 3867–3872.
Welsh M, Welsh N, Bendtzen K, Mares J, Strandell E, Oberg C, Sandler S (1995) Comparison of mRNA contents of interleukin-1β and nitric oxide synthase in pancreatic islets isolated from female and male nonobese diabetic mice. Diabetologia 38: 153–160.
Yamada K, Nonaka K, Hanafusa T, Miyazaki A, Toyoshima H, Tarui S (1982) Preventive and therapeutic effects of large dose nicotinamide injections on diabetes associated with insulitis: an observation in non-obese diabetic (NOD) mice. Diabetes 31: 749–753.
Yamada K, Takane N, Otabe S, Inada C, Inoue M, Nonaka K (1993) Pancreatic β-cell-selective production of tumour necrosis factor-β induced by interleukin-1. Diabetes 42: 1026–1031.
Yamada K, Takane-Gyotoku N, Yuan X, Ichikawa F, Inada C, Nonaka K (1996) Mouse islet cell lysis mediated by interleukin-1-induced Fas. Diabetologia 39: 1306–1312.
Yamagata K, Nakajima H, Tomita K et al. (1996) Dominant TCR α-chain clonotypes and interferon-γ are expressed in the pancreas of patients with recent-onset insulin-dependent diabetes mellitus. Diabetes Res Clin Pract 34: 37–46.
Yonemura Y, Takashima T, Miwa K, Miyazaki T, Yamamoto H, Okamoto H (1984) Amelioration of diabetes mellitus in partially depancreatized rats by poly(ADP-ribose) synthetase inhibitors. Evidence of islet B-cell regeneration. Diabetes 33: 401–404.
Zingarelli B, Salzman AL, Szabo C (1998) Genetic disruption of poly (ADP-ribose) synthetase inhibits the expression of P-selectin and intercellular adhesion molecule-1 in myocardial ischemia/reperfusion injury. Circ Res 83: 85–94.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reddy, S., Young, M. & Ginn, S. Immunoexpression of Interleukin-1β in Pancreatic Islets of NOD Mice during Cyclophosphamide-accelerated Diabetes: Co-localization in Macrophages and Endocrine Cells and its Attenuation with Oral Nicotinamide. Histochem J 33, 317–327 (2001). https://doi.org/10.1023/A:1012422821187
Issue Date:
DOI: https://doi.org/10.1023/A:1012422821187