Abstract
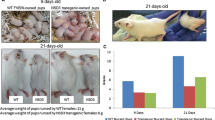
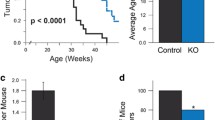
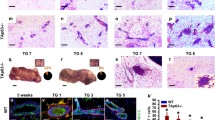
Overexpression of the cyclin D1 oncogene and inactivation of the p53 tumor suppressor have both been implicated in substantial proportions of sporadic human breast cancers. Transgenic mice with cyclin D1 overexpression targeted to mammary tissue by the MMTV enhancer-promoter have been shown to develop mammary cancers. To investigate the relationship between pathways driven by cyclin D1 overexpression and p53 loss during the development of breast cancers, we crossed MMTV-cyclin D1 mice with p53 heterozygous null (p53+/−) mice. In such crossed mice, cyclin D1-driven mammary neoplasia would need to be substantially accelerated by p53 loss in order for mammary tumors to develop prior to the expected onset of non-mammary tumors characteristic of the p53-deficient background alone. Instead, in mice heterozygous or homozygous for p53 deficiency and simultaneously carrying the MMTV-cyclin D1 transgene, only tumors typically found in p53-deficient mice developed and mammary tumors were not observed. Interestingly, MMTV-cyclin D1/p53+/− mice appeared to develop these non-mammary tumors more rapidly than p53+/− mice, and a majority of the sampled non-mammary tumors from MMTV-cyclin D1/p53+/− mice showed ‘ectopic’ expression of the MMTV-driven cyclin D1 transgene. Within the constraints of possible genetic background effects and limited sensitivity due to the early emergence of non-mammary tumors, these observations provide no evidence that inactivation of p53 confers a major additional selective advantage to mammary cells overexpressing cyclin D1 in this animal model of human breast cancer. Interestingly, the results do raise the possibility that p53 inactivation might complement or cooperate with cyclin D1 deregulation during the development of some types of non-mammary tumors.
Similar content being viewed by others
References
Antonescu C, Leung DH, Dudas M, Ladanyi M, Brennan M, Woodruff JM and Cordon-Cardo C (2000) Alterations of cell cycle regulators in localized synovial sarcoma. Am J Pathol 156: 977–983.
Arnold A (1995) The cyclin D1/PRAD1 oncogene in human neoplasia. J Invest Med 43: 543–549.
Bartkova J, Lukas J, Müller H, Lützhφft D, Straus M and Bartek J (1994) Cyclin D1 protein expression and function in human breast cancer. Int J Cancer 57: 353–361.
Bishop JM (1991) Molecular themes in oncogenesis. Cell 64: 235–248.
Buckley MF, Sweeney KJE, Hamilton JA, Sini RL, Manning DL, Nicholson RI, Defazio A, Watts CKW, Musgrove EA and Sutherland RL (1993) Expression and amplification of cyclin genes in human breast cancer. Oncogene 8: 2127–2133.
Bukholm IK, Berner JM, Nesland JM and Borresen-Dale A-L (1998) Expression of cyclin Ds in relation to p53 status in human breast carcinomas. Virchows Arch 433: 223–228.
Canman CE, Gilmar TM, Coutts SB and Kastan MB (1995) Growth factor modulation of p53-mediated growth arrest versus apoptosis. Genes Dev 9: 600–611.
Clarke AR, Purdie CA, Harrison DJ, Morris RG, Bird CC, Hooper ML and Wyllie AH (1993) Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature 352: 849–852.
DePinho R and Jacks T (1999) Introductory Comments. Oncogene 18: 5248–5362.
Donehower LA, Harvey M, Slagle BJ, McArthur MJ, Montgomery CA, Butel JS and Bradley A (1992) Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356: 215–221.
Donehower LA, Godley L A, Aldaz CM, Pyle R, Shi Y-P, Pinkel D, Gray J, Bradley A, Medina D and Varmus HE (1995) Deficiency of p53 accelerates mammary tumorigenesis in Wnt-1 transgenic mice and promote chromosomal instability. Genes Dev 9: 882–895.
Elson A, Deng C, Campos-Torres J, Donehower LA and Leder P (1995) The MMTV/c-myc transgene and p53 null alleles collaborate to induce T-cell lymphomas, but not mammary carcinomas in transgenic mice. Oncogene 11: 181–190.
Gillett C, Fantl V, Smith R, Fisher C, Bartek J, Dickson C, Barnes D and Peters G (1994) Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res 54: 1812–1817.
Greenblatt MS, Bennett WP, Hollstein M and Harris CC (1994) Mutations in the p53 tumor suppressor gene: Clues to cancer etiology and molecular pathogenesis. Cancer Res 54: 4855–4878.
Hansen R, Reddel R and Braithwaite A (1995) The transforming oncoproteins determine the mechanism by which p53 suppresses cell transformation: pRb-mediated growth arrest or apoptosis. Oncogene 11: 2535–2545.
Hogan B, Beddington R, Costantini F and Lacy E (1986) Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Plainview, NY.
Hosokawa Y and Arnold A (1996) Cyclin D1/PRAD1 as a central target in oncogenesis. J Clin Lab Med 127: 246–252.
Hundley JE, Koester SK, Troyer DA, Hilsenbeck SG, Subler MA and Windle JJ (1997) Increased tumor proliferation and genomic instability without decreased apoptosis in MMTV-ras mice deficient in p53. Mol Cell Biol 17: 723–731.
Hunter T (1991) Cooperation between oncogenes. Cell 64: 249–270.
Jerry DJ, Kittrell FS, Kuperwasser C, Laucirica R, Dickinson ES, Bonilla ES, Butel JS and Medina D (2000) A mammary-specific model demonstrates the role of the p53 tumor suppressor gene in tumor development. Oncogene 19: 1052–1058.
Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT and Weinberg RA (1994) Tumor spectrum analysis in p53-mutant mice. Curr Biol 4: 1–7.
Kastan MB, Onyekewere D, Sidransky B, Vogelstein B and Craig RW (1991) Participation of p53 protein in the cellular response to DNA damage. Cancer Res 51: 6304–6311.
Kastan MB, Zhan Q, El-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B and Fornace AJ (1992) A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectiasia. Cell 71: 587–597.
Komatsu H, Iida S, Yamamoto K, Mikuni C, Nitta M, Takahashi T, Ueda R and Seto M (1994) A variant chromosome translocation at 11q13 identifying PRAD1/cyclin D1 as the BCL-1 gene. Blood 84: 1226–1231.
Lammie GA, Fantl V, Smith R, Schuuring E, Brookes S, Michalides R, Dickson C, Arnold A and Peters G (1991) D11S287, a putative oncogene on chromosome 11q13, is amplified and expressed in squamous cell and mammary carcinomas and linked to BCL-1. Oncogene 6: 439–444.
Lin Y and Benchimol S (1995) Cytokines inhibit p53-mediated apoptosis but not p53-mediated G1 arrest. Mol Cell Biol 15: 6045–6054.
Livingstone LR, White A, Sprouse J, Livanos E, Jacks T and Tlsty T (1992) Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell 70: 923–935.
Lowe SW, Schmitt EM, Smith SW, Osborne BA and Jacks T (1993) p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 362: 847–849.
Martin MC, Hsu B, Meyn RE, Donehower LA, El-Naggar AK and McDonnell TJ (1994) Evidence that p53 and bcl-2 are regulators of a common cell death pathway important for in vivo lymphomagenesis. Oncogene 9: 3107–3112.
Michalides R, Hageman P, van Tinteren H, Houben L, Wientjens E, Klompmarker R and Peterse J (1996) A clinicopathological study on overexpression of cyclin D1 and of p53 in a series of 248 patients with operable breast cancer. Br J Cancer 73: 728–734.
Motokura T, Bloom T, Kim HG, Jüppner H, Ruderman JV, Kronenberg HM and Arnold A (1991) A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature 350: 512–515.
Prives C (1998) Signaling to p53: breaking the MDM2-p53 circuit. Cell 95: 5–8.
Rosenberg CL, Wong E, Petty EM, Bale AE, Tsujimoto Y, Harris NL and Arnold A (1991) PRAD1, a candidate BCL1 oncogene: mapping and expression in centrocytic lymphoma. Proc Natl Acad Sci USA 88: 9638–9642.
Seto M, Yamamoto K, Iida S, Akao Y, Utsumi KR, Kubonishi I, Miyoshi T, Ohtsuki T, Yawata Y, Namba M, Motokura T, Arnold A, Takahashi T and Ueda R (1992) Gene rearrangement and overexpression of PRAD1 in lymphoid malignancy with t(11;14)(q13;q32) translocation. Oncogene 7: 1401–1406.
Sherr CJ (1996) Cancer cell cycles. Science 274: 1672–1677.
Siegel PM, Ryan ED, Cardiff RD and Muller WJ (1999) Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. EMBO J 18: 2149–2164.
Suzuki H, Moriya J, Nakahata A, Fujioka Y, Inoue K and Nagashima K (1998) Cyclin D1 gene amplification in esophageal carcinosarcoma shown by differential polymerase chain reaction. Hum Pathol 29: 662–667.
Tanyi J, Tory K, Bankfalvi A, Shroder W, Rath W and Fuzesi L (1999) Analysis of p53 mutation and cyclin D1 expression in breast tumors. Pathol Oncol Res 5: 90–94.
Taubert H, Meye A and Wurl P (1998) Soft tissue sarcomas and p53 mutations. Mol Med 4: 365–372.
Wang TC, Cardiff R, Zukerberg L, Lees E, Arnold A and Schmidt EV (1994) Mammary hyperplasia and carcinoma in MMTVcyclin D1 transgenic mice. Nature 369: 669–671.
Williams ME, Swerdlow SH, Rosenberg CL and Arnold A (1993) Chromosome 11 translocation breakpoints at the PRAD1/cyclin D1 gene locus in centrocytic lymphoma. Leukemia 7: 241–245.
Withers DA, Harvey RC, Faust JB, Melnyk O, Carey K and Meeker TC (1991) Characterization of a candidate bcl1 gene. Mol Cell Biol 11: 4846–4853.
Yin H, Tainsky MA, Bischoff FZ, Strong LC and Wahl GM (1992) Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell 70: 937–948.
Zukerberg LR, Yang W-I, Gadd M, Thor AD, Koerner FC, Schmidt EV and Arnold A (1995) Cyclin D1 (PRAD1) protein expression in breast cancer: approximately one-third of infiltrating mammary carcinomas show overexpression of the cyclin D1 oncogene. Mod Pathol 8: 560–567.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hosokawa, Y., Papanikolaou, A., Cardiff, R.D. et al. In vivo analysis of mammary and non-mammary tumorigenesis in MMTV-cyclin D1 transgenic mice deficient in p53. Transgenic Res 10, 471–478 (2001). https://doi.org/10.1023/A:1012064911751
Issue Date:
DOI: https://doi.org/10.1023/A:1012064911751