Abstract
The β-adrenoceptor (β-AR) mediated signal transduction pathway in cardiomyocytes is known to involve β1- and β2-ARs, stimulatory (Gs) and inhibitory (Gi) guanine nucleotide binding proteins, adenylyl cyclase (AC) and cAMP-dependent protein kinase (PKA). The activation of β1- and β2-ARs has been shown to increase heart function by increasing Ca2+-movements across the sarcolemmal membrane and sarcoplasmic reticulum through the stimulation of Gs-proteins, activation of AC and PKA enzymes and phosphorylation of the target sites. The activation of PKA has also been reported to increase phosphorylation of some myofibrillar proteins (for promoting cardiac relaxation) and nuclear proteins (for cardiac hypertrophy). The activation of β2-AR has also been shown to affect Gi-proteins, stimulate mitogen activated protein kinase and increase protein synthesis by enhancing gene expression. β1- and β2-ARs as well as AC are considered to be regulated by PKA- and protein kinase C (PKC)-mediated phosphorylations directly; both PKA and PKC also regulate β-AR indirectly through the involvement of β-AR kinase (βARK), β-arrestins and Gβγ-protein subunits. Genetic manipulation of different components and regulators of β-AR signal transduction pathway by employing transgenic and knockout mouse models has provided insight into their functional and regulatory characteristics in cardiomyocytes. The genetic studies have also helped in understanding the pathophysiological role of βARK in heart dysfunction and therapeutic role of βARK inhibitors in the treatment of heart failure. Varying degrees of defects in the β-AR signal transduction system have been identified in different types of heart failure to explain the attenuated response of the failing heart to sympathetic stimulation or catecholamine infusion. A decrease in β1-AR density, an increase in the level of Gi-proteins and overexpression of βARK are usually associated with heart failure; however, these attenuations have been shown to be dependent upon the type and stage of heart failure as well as region of the heart. Both local and circulating renin-angiotensin systems, sympathetic nervous system and endothelial cell function appears to regulate the status of β-AR signal transduction pathway in the failing heart. Thus different components and regulators of the β-AR signal transduction pathway appears to represent important targets for the development of therapeutic interventions for the treatment of heart failure.
Similar content being viewed by others
References
Opie LH: Receptors and signal transductions. In: The Heart: Physiology, from Cell to Circulation. Lippincott-Raven Publishers, Philadephia, 1998, pp 173–207
Omens JH, Covell JW: Transmural distribution of myocardial tissue growth induced by volume-overload hypertrophy in the dog. Circulation 84: 1235–1245, 1991
Bristow MR, Hershberger RE, Port JD, Gilbert EM, Sandoval A, Rasmussen R, Cates AE, Feldman AM: β-adrenergic pathways in nonfailing and failing human ventricular myocardium. Circulation 82: I12–I25, 1990
Brodde OE, Hillemann S, Kunde K, Vogelsang M, Zerkowski HR: Receptor systems affecting force of contraction in the human heart and their alterations in chronic heart failure. J Heart Lung Transplant 11: S164–S174, 1992
Bristow MR, Port JD, Hershberger RE, Gilbert EM, Feldman AM: The β-adrenergic receptor-adenylate cyclase complex as a target for therapeutic intervention in heart failure. Eur Heart J 10(suppl B): 45–54, 1989
Lefkowitz RJ: G protein-coupled receptors. III. New roles for receptor kinases and β-arrestins in receptor signaling and desensitization. J Biol Chem 273: 18677–18680, 1998
Vatner SF, Vatner DE, Homcy CJ: β-adrenergic receptor signaling: An acute compensatory adjustment — inappropriate for the chronic stress of heart failure? Insights from Gsα overexpression and other genetically engineered animal models. Circ Res 86: 502–506, 2000
Drazner MH, Koch WJ, Lefkowitz RJ: Potentiation of β-adrenergic signaling by gene transfer. Proc Assoc Am Phys 109: 220–227, 1997
Koch WJ, Milano CA, Lefkowitz RJ: Transgenic manipulation of myocardial G protein-coupled receptors and receptor kinases. Circ Res 78: 511–516, 1996
Koch WJ, Lefkowitz RJ, Milano CA, Akhter SA, Rockman HA: Myocardial overexpression of adrenergic receptors and receptor kinases. Adv Pharmacol 42: 502–506, 1998
Kaumann AJ, Molenaar P: Modulation of human cardiac function through 4 β-adrenoceptor populations. Naunyn Schmiedebergs Arch Pharmacol 355: 667–681, 1997
Tate KM, Briend-Sutren MM, Emorine LJ, Delavier-Klutchko C, Marullo S, Strosberg AD: Expression of three human β-adrenergic-receptor subtypes in transfected Chinese hamster ovary cells. Eur J Biochem 196: 357–361, 1991
Frielle T, Collins S, Daniel KW, Caron MG, Lefkowitz RJ, Kobilka BK: Cloning of the cDNA for the human β1-adrenergic receptor. Proc Natl Acad Sci USA 84: 7920–7924, 1987
Kobilka BK, Dixon RA, Frielle T, Dohlman HG, Bolanowski MA, Sigal IS, Yang-Feng TL, Francke U, Caron MG, Lefkowitz RJ: cDNA for the human β2-adrenergic receptor: A protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc Natl Acad Sci USA 84: 46–50, 1987
Machida CA, Bunzow JR, Searles RP, Van Tol H, Tester B, Neve KA, Teal P, Nipper V, Civelli O: Molecular cloning and expression of the rat β1-adrenergic receptor gene. J Biol Chem 265: 12960–12965, 1990
Rubenstein RC, Wong SK, Ross EM: The hydrophobic tryptic core of the β-adrenergic receptor retains Gs regulatory activity in response to agonists and thiols. J Biol Chem 262: 16655–16662, 1987
Emorine LJ, Marullo S, Briend-Sutren MM, Patey G, Tate K, Delavier-Klutchko C, Strosberg AD: Molecular characterization of the human β3-adrenergic receptor. Science 245: 1118–1121, 1989
Tota MR, Candelore MR, Dixon RA, Strader CD: Biophysical and genetic analysis of the ligand-binding site of the β-adrenoceptor. Trends Pharmacol Sci 12: 4–6, 1991
Dohlman HG, Caron MG, Strader CD, Amlaiky N, Lefkowitz RJ: Identification and sequence of a binding site peptide of the β2-adrenergic receptor. Biochemistry 27: 1813–1817, 1988
Kobilka BK, Kobilka TS, Daniel K, Regan JW, Caron MG, Lefkowitz RJ: Chimeric α2-, β2-adrenergic receptors: Delineation of domains involved in effector coupling and ligand binding specificity. Science 240: 1310–1316, 1988
Strader CD, Dixon RA, Cheung AH, Candelore MR, Blake AD, Sigal IS: Mutations that uncouple the β-adrenergic receptor from Gs and increase agonist affinity. J Biol Chem 262: 16439–16443, 1987
Benovic JL, Pike LJ, Cerione RA, Staniszewski C, Yoshimasa T, Codina J, Caron MG, Lefkowitz RJ: Phosphorylation of the mammalian β-adrenergic receptor by cyclic AMP-dependent protein kinase. Regulation of the rate of receptor phosphorylation and dephosphorylation by agonist occupancy and effects on coupling of the receptor to the stimulatory guanine nucleotide regulatory protein. J Biol Chem 260: 7094–7101, 1985
Benovic JL, Strasser RH, Caron MG, Lefkowitz RJ: β-adrenergic receptor kinase: Identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci USA 83: 2797–2801, 1986
Cheung AH, Sigal IS, Dixon RA, Strader CD: Agonist-promoted sequestration of the β2-adrenergic receptor requires regions involved in functional coupling with Gs. Mol Pharmacol 35: 132–138, 1989
O'Dowd BF, Hnatowich M, Regan JW, Leader WM, Caron MG, Lefkowitz RJ: Site-directed mutagenesis of the cytoplasmic domains of the human β2-adrenergic receptor. Localization of regions involved in G protein-receptor coupling. J Biol Chem 263: 15985–15992, 1988
Strosberg AD: Structural and functional diversity of β-adrenergic receptors. Ann N Y Acad Sci 757: 253–260, 1995
Raymond JR, Hnatowich M, Lefkowitz RJ, Caron MG: Adrenergic receptors. Models for regulation of signal transduction processes. Hypertension 15: 119–131, 1990
Brodde OE: β1-and β2-adrenoceptors in the human heart: Properties, function, and alterations in chronic heart failure. Pharmacol Rev 43: 203–242, 1991
Bristow MR, Ginsburg R: β2 receptors on myocardial cells in human ventricular myocardium. Am J Cardiol 57: 3F–6F, 1986
Gauthier C, Tavernier G, Charpentier F, Langin D, Le Marec H: Functional β3-adrenoceptor in the human heart. J Clin Invest 98: 556–562, 1996
Krief S, Lonnqvist F, Raimbault S, Baude B, van Spronsen A, Arner P, Strosberg AD, Ricquier D, Emorine LJ: Tissue distribution of β3-adrenergic receptor mRNA in man. J Clin Invest 91: 344–349, 1993
Kaumann AJ, Morris TH, Bojar H: Heart β-receptors: On the functional role of heterogeneous binding sites. J Receptor Res 3: 61–70, 1983
Kaumann AJ: Is there a third heart β-adrenoceptor? Trends Pharmacol Sci 10: 316–320, 1989
Kaumann AJ, Molenaar P: Differences between the third cardiac badrenoceptor and the colonic β3-adrenoceptor in the rat. Br J Pharmacol 118: 2085–2098, 1996
Kaumann AJ: (–)-CGP 12177-induced increase of human atrial contraction through a putative third β-adrenoceptor. Br J Pharmacol 117: 93–98, 1996
Lafontan M, Barbe P, Galitzky J, Tavernier G, Langin D, Carpene C, Bousquet-Melou A, Berlan M: Adrenergic regulation of adipocyte metabolism. Hum Reprod 12(suppl 1): 6–20, 1997
Xiao RP, Cheng H, Zhou YY, Kuschel M, Lakatta EG: Recent advances in cardiac β2-adrenergic signal transduction. Circ Res 85: 1092–1100, 1999
Daaka Y, Luttrell LM, Lefkowitz RJ: Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature 390: 88–91, 1997
Begin-Heick N: β3-adrenergic activation of adenylyl cyclase in mouse white adipocytes: Modulation by GTP and effect of obesity. J Cell Biochem 58: 464–473, 1995
Chaudhry A, MacKenzie RG, Georgic LM, Granneman JG: Differential interaction of β1-and β3-adrenergic receptors with Gi in rat adipocytes. Cell Signal 6: 457–465, 1994
Kaumann AJ, Birnbaumer L: Adrenergic receptors in heart muscle: Similarity of apparent affinities of β-blockers for receptors mediating adenyl-cyclase activity, inotropic and chronotropic effects of catecholamines. Acta Physiol Lat Am 23: 619–620, 1973
Malinowska B, Schlicker E: Mediation of the positive chronotropic effect of CGP 12177 and cyanopindolol in the pithed rat by atypical β-adrenoceptors, different from β3-adrenoceptors. Br J Pharmacol 117: 943–949, 1996
Gilman AG: G proteins: Transducers of receptor-generated signals. Annu Rev Biochem 56: 615–649, 1987
Casey PJ, Gilman AG: G protein involvement in receptor-effector coupling. J Biol Chem 263: 2577–2580, 1988
Jones DT, Reed RR: Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem 262: 14241–14249, 1987
Ray K, Kunsch C, Bonner LM, Robishaw JD: Isolation of cDNA clones encoding eight different human G protein δ subunits, including three novel forms designated the δ4, δ10, and δ11 subunits. J Biol Chem 270: 21765–21771, 1995
Simon MI, Strathmann MP, Gautam N: Diversity of G proteins in signal transduction. Science 252: 802–808, 1991
Watson AJ, Aragay AM, Slepak VZ, Simon MI: A novel form of the G protein β subunit Gβ5 is specifically expressed in the vertebrate retina. J Biol Chem 271: 28154–28160, 1996
Johnson MD, Friedman E: G proteins in cardiovascular function and dysfunction. Biochem Pharmacol 45: 2365–2372, 1993
Holmer SR, Stevens S, Homcy CJ: Tissue-and species-specific expression of inhibitory guanine nucleotide-binding proteins. Cloning of a full-length complementary DNA from canine heart. Circ Res 65: 1136–1140, 1989
Luetje CW, Tietje KM, Christian JL, Nathanson NM: Differential tissue expression and developmental regulation of guanine nucleotide binding regulatory proteins and their messenger RNAs in rat heart. J Biol Chem 263: 13357–13365, 1988
Hansen CA, Schroering AG, Robishaw JD: Subunit expression of signal transducing G proteins in cardiac tissue: Implications for phospholipase C-β regulation. J Mol Cell Cardiol 27: 471–484, 1995
Tang WJ, Gilman AG: Type-specific regulation of adenylyl cyclase by G protein β, δ subunits. Science 254: 1500–1503, 1991
Taussig R, Iniguez-Lluhi JA, Gilman AG: Inhibition of adenylyl cyclase by Giα. Science 261: 218–221, 1993
Taussig R, Tang WJ, Hepler JR, Gilman AG: Distinct patterns of bidirectional regulation of mammalian adenylyl cyclases. J Biol Chem 269: 6093–6100, 1994
Clapham DE, Neer EJ: G protein βγ subunits. Annu Rev Pharmacol Toxicol 37: 167–203, 1997
Muller S, Hekman M, Lohse MJ: Specific enhancement of β-adrenergic receptor kinase activity by defined G-protein β and δ subunits. Proc Natl Acad Sci USA 90: 10439–10443, 1993
Sunahara RK, Dessauer CW, Gilman AG: Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol 36: 461–480, 1996
Iyengar R: Molecular and functional diversity of mammalian Gs-stimulated adenylyl cyclases. FASEB J 7: 768–775, 1993
Krupinski J, Coussen F, Bakalyar HA, Tang WJ, Feinstein PG, Orth K, Slaughter C, Reed RR, Gilman AG: Adenylyl cyclase amino acid sequence: Possible channel-or transporter-like structure. Science 244: 1558–1564, 1989
Cali JJ, Zwaagstra JC, Mons N, Cooper DM, Krupinski J: Type VIII adenylyl cyclase. A Ca2+/calmodulin-stimulated enzyme expressed in discrete regions of rat brain. J Biol Chem 269: 12190–12195, 1994
Tang WJ, Krupinski J, Gilman AG: Expression and characterization of calmodulin-activated (type I) adenylyl cyclase. J Biol Chem 266: 8595–8603, 1991
Katsushika S, Chen L, Kawabe J, Nilakantan R, Halnon NJ, Homcy CJ, Ishikawa Y: Cloning and characterization of a sixth adenylyl cyclase isoform: Types V and VI constitute a subgroup within the mammalian adenylyl cyclase family. Proc Natl Acad Sci USA 89: 8774–8778, 1992
Wallach J, Droste M, Kluxen FW, Pfeuffer T, Frank R: Molecular cloning and expression of a novel type V adenylyl cyclase from rabbit myocardium. FEBS Lett 338: 257–263, 1994
Yoshimura M, Cooper DM: Cloning and expression of a Ca2+-inhibitable adenylyl cyclase from NCB-20 cells. Proc Natl Acad Sci USA 89: 6716–6720, 1992
Ping P, Anzai T, Gao M, Hammond HK: Adenylyl cyclase and G protein receptor kinase expression during development of heart failure. Am J Physiol 273: H707–H717, 1997
Premont RT, Matsuoka I, Mattei MG, Pouille Y, Defer N, Hanoune J: Identification and characterization of a widely expressed form of adenylyl cyclase. J Biol Chem 271: 13900–13907, 1996
Ishikawa Y, Katsushika S, Chen L, Halnon NJ, Kawabe J, Homcy CJ: Isolation and characterization of a novel cardiac adenylyl cyclase cDNA. J Biol Chem 267: 13553–13557, 1992
Yu HJ, Unnerstall JR, Green RD: Determination and cellular localization of adenylyl cyclase isozymes expressed in embryonic chick heart. FEBS Lett 374: 89–94, 1995
Watanabe AM, McConnaughey MM, Strawbridge RA, Fleming JW, Jones LR, Besch HR Jr: Muscarinic cholinergic receptor modulation of beta-adrenergic receptor affinity for catecholamines. J Biol Chem 253: 4833–4836, 1978
Lefkowitz RJ, Pitcher J, Krueger K, Daaka Y: Mechanisms of betaadrenergic receptor desensitization and resensitization. Adv Pharmacol 42: 416–420, 1998
Inglese J, Freedman NJ, Koch WJ, Lefkowitz RJ: Structure and mechanism of the G protein-coupled receptor kinases. J Biol Chem 268: 23735–23738, 1993
Premont RT, Inglese J, Lefkowitz RJ: Protein kinases that phosphorylate activated G protein-coupled receptors. FASEB J 9: 175–182, 1995
Pitcher JA, Touhara K, Payne ES, Lefkowitz RJ: Pleckstrin homology domain-mediated membrane association and activation of the β-adrenergic receptor kinase requires coordinate interaction with G βδ subunits and lipid. J Biol Chem 270: 11707–11710, 1995
Carman CV, Barak LS, Chen C, Liu-Chen LY, Onorato JJ, Kennedy SP, Caron MG, Benovic JL: Mutational analysis of Gβδ and phospholipid interaction with G protein-coupled receptor kinase 2. J Biol Chem 275: 10443–10452, 2000
Ferguson SS, Menard L, Barak LS, Koch WJ, Colapietro AM, Caron MG: Role of phosphorylation in agonist-promoted β2-adrenergic receptor sequestration. Rescue of a sequestration-defective mutant receptor by β ARK1. J Biol Chem 270: 24782–24789, 1995
Ferguson SS, Downey WE, Colapietro AM, Barak LS, Menard L, Caron MG: Role of β-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science 271: 363–366, 1996
Goodman OB Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL: β-arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature 383: 447–450, 1996
Zhang J, Ferguson SG, Barak LS, Menard L, Caron MG: Dynamin and β-arrestin reveal distinct mechanisms for G protein-coupled receptor internalization. J Biol Chem 271: 18302–18305, 1996
Gagnon AW, Kallal L, Benovic JL: Role of clathrin-mediated endocytosis in agonist-induced down-regulation of the β2-adrenergic receptor. J Biol Chem 273: 6976–6981, 1998
Danner S, Lohse MJ: Cell type-specific regulation of β2-adrenoceptor mRNA by agonists. Eur J Pharmacol 331: 73–78, 1997
Bouvier M, Collins S, O'Dowd BF, Campbell PT, de Blasi A, Kobilka BK, MacGregor C, Irons GP, Caron MG, Lefkowitz RJ: Two distinct pathways for cAMP-mediated down-regulation of the β2-adrenergic receptor. Phosphorylation of the receptor and regulation of its mRNA level. J Biol Chem 264: 16786–16792, 1989
Hadcock JR, Malbon CC: Down-regulation of β-adrenergic receptors: agonist-induced reduction in receptor mRNA levels. Proc Natl Acad Sci USA 85: 5021–5025, 1988
Hausdorff WP, Caron MG, Lefkowitz RJ: Turning off the signal: Desensitization of β-adrenergic receptor function. FASEB J 4: 2881–2889, 1990
Pitcher JA, Payne ES, Csortos C, DePaoli-Roach AA, Lefkowitz RJ: The G-protein-coupled receptor phosphatase: A protein phosphatase type 2A with a distinct subcellular distribution and substrate specificity. Proc Natl Acad Sci USA 92: 8343–8347, 1995
Krueger KM, Daaka Y, Pitcher JA, Lefkowitz RJ: The role of sequestration in G protein-coupled receptor resensitization. Regulation of β2-adrenergic receptor dephosphorylation by vesicular acidification. J Biol Chem 272: 5–8, 1997
Bouvier M, Rousseau G: Subtype-specific regulation of the β-adrenergic receptors. Adv Pharmacol 42: 433–438, 1998
Levis MJ, Bourne HR: Activation of the α subunit of Gs in intact cells alters its abundance, rate of degradation, and membrane avidity. J Cell Biol 119: 1297–1307, 1992
Wedegaertner PB, Bourne HR: Activation and depalmitoylation of Gsα. Cell 77: 1063–1070, 1994
Bertin B, Mansier P, Makeh I, Briand P, Rostene W: Specific atrial overexpression of G-protein coupled human β1 adrenoceptors in transgenic mice. Cardiovasc Res 27: 1606–1612, 1993
Mansier P, Medigue C, Charlotte N, Vernerien C, Coraboeuf E: Decreased heart rate variability in transgenic mice overexpressing atrial β1-adrenoceptors. Am J Physiol 271: H1465–H1472, 1996
Pelzer S, Shuba YM, Asai T, Codina J, Birnbaumer L, McDonald TF, Pelzer D: Membrane-delimited stimulation of heart cell calcium current by β-adrenergic signal-transducing Gs protein. Am J Physiol 259: H264–H267, 1990
Yatani A, Brown AM: Rapid β-adrenergic modulation of cardiac calcium channel currents by a fast G protein pathway. Science 245: 71–74, 1989
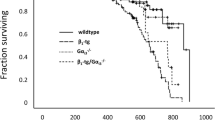
Engelhardt S, Hein L, Wiesmann F, Lohse MJ: Progressive hypertrophy and heart failure in β1-adrenergic receptor transgenic mice. Proc Natl Acad Sci USA 96: 7059–7064, 1999
Milano CA, Allen LF, Rockman HA, Dolber PC, McMinn TR, Chien KR, Johnson TD, Bond RA, Lefkowitz RJ: Enhanced myocardial function in transgenic mice overexpressing the β2-adrenergic receptor. Science 264: 582–586, 1994
Samama P, Cotecchia S, Costa T, Lefkowitz RJ: A mutation-induced activated state of the β2-adrenergic receptor. Extending the ternary complex model. J Biol Chem 268: 4625–4636, 1993
Bond RA, Leff P, Johnson TD, Milano CA, Rockman HA, McMinn TR, Apparsundaram S, Hyek MF, Kenakin TP, Allen LF: Physiological effects of inverse agonists in transgenic mice with myocardial overexpression of the β2-adrenoceptor. Nature 374: 272–276, 1995
Levy FO, Zhu X, Kaumann AJ, Birnbaumer L: Efficacy of β1-adrenergic receptors is lower than that of β2-adrenergic receptors. Proc Natl Acad Sci USA 90: 10798–10802, 1993
Arber S, Hunter JJ, Ross J Jr, Hongo M, Sansig G, Borg J, Perriard JC, Chien KR, Caroni P: MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell 88: 393–403, 1997
Rockman HA, Chien KR, Choi DJ, Iaccarino G, Hunter JJ, Ross J Jr, Lefkowitz RJ, Koch WJ: Expression of a β-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc Natl Acad Sci USA 95: 7000–7005, 1998
Du XJ, Autelitano DJ, Dilley RJ, Wang B, Dart AM, Woodcock EA: β2-adrenergic receptor overexpression exacerbates development of heart failure after aortic stenosis. Circulation 101: 71–77, 2000
Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML: Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med 325: 1468–1475, 1991
Roubin GS, Choong CY, Devenish-Meares S, Sadick NN, Fletcher PJ, Kelly DT, Harris PJ: β-adrenergic stimulation of the failing ventricle: A double-blind, randomized trial of sustained oral therapy with prenalterol. Circulation 69: 955–962, 1984
Rohrer DK, Desai KH, Jasper JR, Stevens ME, Regula DP Jr, Barsh GS, Bernstein D, Kobilka BK: Targeted disruption of the mouse beta1-adrenergic receptor gene: Developmental and cardiovascular effects. Proc Natl Acad Sci USA 93: 7375–7380, 1996
Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, Kobilka BK: Targeted disruption of the β2-adrenergic receptor gene. J Biol Chem 274: 16694–16700, 1999
Messerli FH, Weber MA, Brunner HR: Angiotensin II receptor inhibition. A new therapeutic principle. Arch Intern Med 156: 1957–1965, 1996
Susulic VS, Frederich RC, Lawitts J, Tozzo E, Kahn BB, Harper ME, Himms-Hagen J, Flier JS, Lowell BB: Targeted disruption of the β3-adrenergic receptor gene. J Biol Chem 270: 29483–29492, 1995
Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, Lefkowitz RJ: Cardiac function in mice overexpressing the badrenergic receptor kinase or a β ARK inhibitor. Science 268: 1350–1353, 1995
Brink M, Wellen J, Delafontaine P: Angiotensin II causes weight loss and decreases circulating insulin-like growth factor I in rats through a pressor-independent mechanism. J Clin Invest 97: 2509–2516, 1996
Rockman HA, Choi DJ, Rahman NU, Akhter SA, Lefkowitz RJ, Koch WJ: Receptor-specific in vivo desensitization by the G protein-coupled receptor kinase-5 in transgenic mice. Proc Natl Acad Sci USA 93: 9954–9959, 1996
Oppermann M, Freedman NJ, Alexander RW, Lefkowitz RJ: Phosphorylation of the type 1A angiotensin II receptor by G proteincoupled receptor kinases and protein kinase C. J Biol Chem 271: 13266–13272, 1996
Jaber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, Ross J, Lefkowitz RJ, Caron MG, Giros B: Essential role of β-adrenergic receptor kinase 1 in cardiac development and function. Proc Natl Acad Sci USA 93: 12974–12979, 1996
Rockman HA, Choi DJ, Akhter SA, Jaber M, Giros B, Lefkowitz RJ, Caron MG, Koch WJ: Control of myocardial contractile function by the level of β-adrenergic receptor kinase 1 in gene-targeted mice. J Biol Chem 273: 18180–18184, 1998
Akhter SA, Skaer CA, Kypson AP, McDonald PH, Peppel KC, Glower DD, Lefkowitz RJ, Koch WJ: Restoration of β-adrenergic signaling in failing cardiac ventricular myocytes via adenoviral-mediated gene transfer. Proc Natl Acad Sci USA 94: 12100–12105, 1997
Schmitz W, Boknik P, Linck B, Muller FU: Adrenergic and muscarinic receptor regulation and therapeutic implications in heart failure. Mol Cell Biochem 157: 251–258, 1996
Gaudin C, Ishikawa Y, Wight DC, Mahdavi V, Nadal GB, Wagner TE, Vatner DE, Homcy CJ: Overexpression of Gsα protein in the hearts of transgenic mice. J Clin Invest 95: 1676–1683, 1995
Iwase M, Bishop SP, Uechi M, Vatner DE, Shannon RP, Kudej RK, Wight DC, Wagner TE, Ishikawa Y, Homcy CJ, Vatner SF: Adverse effects of chronic endogenous sympathetic drive induced by cardiac Gsα overexpression. Circ Res 78: 517–524, 1996
Asai K, Yang GP, Geng YJ, Takagi G, Bishop S, Ishikawa Y, Shannon RP, Wagner TE, Vatner DE, Homcy CJ, Vatner SF: β-adrenergic receptor blockade arrests myocyte damage and preserves cardiac function in the transgenic Gsα mouse. J Clin Invest 104: 551–558, 1999
Tepe NM, Lorenz JN, Yatani A, Dash R, Kranias EG, Dorn GW, Liggett SB: Altering the receptor-effector ratio by transgenic overexpression of type V adenylyl cyclase: Enhanced basal catalytic activity and function without increased cardiomyocyte β-adrenergic signalling. Biochemistry 38: 16706–16713, 1999
Gao MH, Lai NC, Roth DM, Zhou J, Zhu J, Anzai T, Dalton N, Hammond HK: Adenylyl cyclase increases responsiveness to catecholamine stimulation in transgenic mice. Circulation 99: 1618–1622, 1999
Post SR, Hilal DR, Urasawa K, Brunton LL, Insel PA: Quantification of signalling components and amplification in the β-adrenergic-receptor-adenylate cyclase pathway in isolated adult rat ventricular myocytes. Biochem J 311: 75–80, 1995
Iwase M, Uechi M, Vatner DE, Asai K, Shannon RP, Kudej RK, Wagner TE, Wight DC, Patrick TA, Ishikawa Y, Homcy CJ, Vatner SF: Cardiomyopathy induced by cardiac Gsα overexpression. Am J Physiol 272: H585–H589, 1997
Gao M, Ping P, Post S, Insel PA, Tang R, Hammond HK: Increased expression of adenylyl cyclase type VI proportionately increases β-adrenergic receptor-stimulated production of cAMP in neonatal rat cardiac myocytes. Proc Natl Acad Sci USA 95: 1038–1043, 1998
Roth DM, Gao MH, Lai NC, Drumm J, Dalton N, Zhou JY, Zhu J, Entrikin D, Hammond HK: Cardiac-directed adenylyl cyclase expression improves heart function in murine cardiomyopathy. Circulation 99: 3099–3102, 1999
Wei LN: Transgenic animals as new approaches in pharmacological studies. Annu Rev Pharmacol Toxicol 37: 119–141, 1997
Furth PA, St Onge L, Boger H, Gruss P, Gossen M, Kistner A, Bujard H, Hennighausen L: Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc Natl Acad Sci USA 91: 9302–9306, 1994
Gu H, Marth JD, Orban PC, Mossmann H, Rajewsky K: Deletion of a DNA polymerase β gene segment in T cells using cell type-specific gene targeting. Science 265: 103–106, 1994
Kuhn R, Schwenk F, Aguet M, Rajewsky K: Inducible gene targeting in mice. Science 269: 1427–1429, 1995
Braunwald E: In: Heart Disease. A Textbook of Cardiovascular Medicine. Philadelphia, 1997
Dhalla NS, Afzal N, Beamish RE, Naimark B, Takeda N, Nagano M: Pathophysiology of cardiac dysfunction in congestive heart failure. Can J Cardiol 9: 873–887, 1993
Cohn JN: Sympathetic nervous system activity and the heart. Am J Hypertens 2: 353S–356S, 1989
Packer M, Lee WH, Kessler PD, Gottlieb SS, Bernstein JL, Kukin ML: Role of neurohormonal mechanisms in determining survival in patients with severe chronic heart failure. Circulation 75: IV80–IV92, 1987
Cohn JN: Plasma norepinephrine and mortality. Clin Cardiol 18: I9–I12, 1995
Packer M: Modulation of functional capacity and survival in congestive heart failure. Effects of activation of the sympathetic nervous system. Postgrad Med Spec No: 96–103, 1988
Post SR, Hammond HK, Insel PA: β-adrenergic receptors and receptor signaling in heart failure. Annu Rev Pharmacol Toxicol 39: 343–360, 1999
Vatner DE, Asai K, Iwase M, Ishikawa Y, Shannon RP, Homcy CJ, Vatner SF: β-adrenergic receptor-G protein-adenylyl cyclase signal transduction in the failing heart. Am J Cardiol 83: 80H–85H, 1999
Dhalla NS, Wang X, Sethi R, Das PK, Beamish RE: β-adrenergic linked signal transduction mechanisms in failing hearts. Heart Failure Rev 2: 55–65, 1997
Flesch M, Erdmann E, Bohm M: Changes in β-adrenoceptors and Gproteins during the transition from cardiac hypertrophy to heart failure. J Cardiac Failure 2: S35–S43, 1996
Choi DJ, Rockman HA: β-adrenergic receptor desensitization in cardiac hypertrophy and heart failure. Cell Biochem Biophys 31: 321–329, 1999
Brodde OE: β-adrenergic receptors in failing human myocardium. Basic Res Cardiol 91(suppl 2): 35–40, 1996
Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB: Decreased catecholamine sensitivity and β-adrenergic-receptor density in failing human hearts. N Engl J Med 307: 205–211, 1982
Bohm M, Beuckelmann D, Brown L, Feiler G, Lorenz B, Nabauer M, Kemkes B, Erdmann E: Reduction of β-adrenoceptor density and evaluation of positive inotropic responses in isolated, diseased human myocardium. Eur Heart J 9: 844–852, 1988
Calderone A, Bouvier M, Li K, Juneau C, de Champlain J, Rouleau JL: Dysfunction of the β-and α-adrenergic systems in a model of congestive heart failure. The pacing-overdrive dog. Circ Res 69: 332–343, 1991
Cartagena G, Sapag HM, Jalil J, Tapia V, Guarda E, Foncea R, Corbalan R, Ebensperger R, Lavandero S: Changes in β-adrenergic receptors of rat heart and adipocytes during volume-overload induced cardiac hypertrophy. Int J Clin Pharmacol Ther Toxicol 31: 198–203, 1993
Pela G, Missale C, Raddino R, Condorelli E, Spano PF, Visioli O: β1-and β2-receptors are differentially desensitized in an experimental model of heart failure. J Cardiovasc Pharmacol 16: 839–846, 1990
Steinfath M, Geertz B, Schmitz W, Scholz H, Haverich A, Breil I, Hanrath P, Reupcke C, Sigmund M, Lo HB: Distinct down-regulation of cardiac β1-and β2-adrenoceptors in different human heart diseases. Naunyn Schmiedebergs Arch Pharmacol 343: 217–220, 1991
Sullebarger JT, Fan TH, Torres F, Liang CS: Both cell surface and internalized β-adrenoceptors are reduced in the failing myocardium. Eur J Pharmacol 205: 165–169, 1991
Bristow MR, Kantrowitz NE, Ginsburg R, Fowler MB: β-adrenergic function in heart muscle disease and heart failure. J Mol Cell Cardiol 17(suppl 2): 41–52, 1985
Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S, Stinson EB: β1-and β2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: Coupling of both receptor subtypes to muscle contraction and selective β1-receptor down-regulation in heart failure. Circ Res 59: 297–309, 1986
Bristow MR, Sandoval AB, Gilbert EM, Deisher T, Minobe W, Rasmussen R: Myocardial α-and β-adrenergic receptors in heart failure: Is cardiac-derived norepinephrine the regulatory signal? Eur Heart J 9(suppl H): 35–40, 1988
Bristow MR, Hershberger RE, Port JD, Minobe W, Rasmussen R: β1-and β2-adrenergic receptor-mediated adenylate cyclase stimulation in nonfailing and failing human ventricular myocardium. Mol Pharmacol 35: 295–303, 1989
Bristow MR, Feldman AM: Changes in the receptor-G proteinadenylyl cyclase system in heart failure from various types of heart muscle disease. Basic Res Cardiol 87(suppl 1): 15–35, 1992
Bristow MR, Minobe W, Rasmussen R, Larrabee P, Skerl L, Klein JW, Anderson FL, Murray J, Mestroni L, Karwande SV: β-adrenergic neuroeffector abnormalities in the failing human heart are produced by local rather than systemic mechanisms. J Clin Invest 89: 803–815, 1992
Altschuld RA, Starling RC, Hamlin RL, Billman GE, Hensley J, Castillo L, Fertel RH, Hohl CM, Robitaille PM, Jones LR: Response of failing canine and human heart cells to β2-adrenergic stimulation. Circulation 92: 1612–1618, 1995
Kaura D, Takeda N, Sethi R, Wang X, Nagano M, Dhalla NS: β-adrenoceptor mediated signal transduction in congestive heart failure in cardiomyopathic (UM-X7.1) hamsters. Mol Cell Biochem 157: 191–196, 1996
Sakagoshi N, Nakano S, Taniguchi K, Hirata N, Matsuda H: Relation between myocardial β-adrenergic receptor and left ventricular function in patients with left ventricular volume overload due to chronic mitral regurgitation with or without aortic regurgitation. Am J Cardiol 68: 81–84, 1991
Ohsuzu F, Katsushika S, Akanuma M, Hakamada N, Hamada H, Maie S, Muneoka K, Okumori M, Nakamura H: Reduction of β-adrenergic receptors in atrial cell membranes of patients following mild to moderate heart failure. Jpn Heart J 35: 27–34, 1994
Sethi R, Bector N, Takeda N, Nagano M, Jasmin G, Dhalla NS: Alterations in G-proteins in congestive heart failure in cardiomyopathic (UM-X7.1) hamsters. Mol Cell Biochem 140: 163–170, 1994
Witte K, Schnecko A, Olbrich HG, Lemmer B: Efficiency of badrenoceptor subtype coupling to cardiac adenylyl cyclase in cardiomyopathic and control hamsters. Eur J Pharmacol 290: 1–10, 1995
Larosa G, Armstrong PW, Seeman P, Forster C: β adrenoceptor recovery after heart failure in the dog. Cardiovasc Res 27: 489–493, 1993
Kiuchi K, Shannon RP, Komamura K, Cohen DJ, Bianchi C, Homcy CJ, Vatner SF, Vatner DE: Myocardial β-adrenergic receptor function during the development of pacing-induced heart failure. J Clin Invest 91: 907–914, 1993
Gilbert EM, Olsen SL, Renlund DG, Bristow MR: β-adrenergic receptor regulation and left ventricular function in idiopathic dilated cardiomyopathy. Am J Cardiol 71: 23C–29C, 1993
Brodde OE: Pathophysiology of the β-adrenoceptor system in chronic heart failure: Consequences for treatment with agonists, partial agonists or antagonists? Eur Heart J 12(suppl F): 54–62, 1991
Steinfath M, Lavicky J, Schmitz W, Scholz H, Doring V, Kalmar P: Regional distribution of β1-and β2-adrenoceptors in the failing and nonfailing human heart. Eur J Clin Pharmacol 42: 607–611, 1992
Schwinger RH, Bohm M, Pieske B, Erdmann E: Different β-adrenoceptor-effector coupling in human ventricular and atrial myocardium. Eur J Clin Invest 21: 443–451, 1991
Beau SL, Saffitz JE: Transmural heterogeneity of norepinephrine uptake in failing human hearts. J Am Coll Cardiol 23: 579–585, 1994
Beau SL, Tolley TK, Saffitz JE: Heterogeneous transmural distribution of β-adrenergic receptor subtypes in failing human hearts. Circulation 88: 2501–2509, 1993
Yoshie H, Tobise K, Onodera S: Intraventricular changes in the β-adrenoceptor-adenylate cyclase system of the rat heart with the progress of monocrotaline-induced right ventricular hypertrophy. Jpn Circ J 58: 855–865, 1994
Tawarahara K, Kurata C, Taguchi T, Kobayashi A, Yamazaki N: Augmentation of β adrenergic receptors in cardiomyopathic hamsters (BIO 14.6) with heart failure. Cardiovasc Res 26: 526–533, 1992
Pitschner HF, Droege A, Mitze M, Schlepper M, Brodde OE: Downregulated β-adrenoceptors in severely failing human ventricles: Uniform regional distribution, but no increased internalization. Basic Res Cardiol 88: 179–191, 1993
Bohm M, Gierschik P, Knorr A, Larisch K, Weismann K, Erdmann E: Role of altered G-protein expression in the regulation of myocardial adenylate cyclase activity and force of contraction in spontaneous hypertensive cardiomyopathy in rats. J Hypertens 10: 1115–1128, 1992
Chasteney EA, Liang CS, Hood WB Jr: β-adrenoceptor and adenylate cyclase function in the infarct model of rat heart failure. Proc Soc Exp Biol Med 200: 90–94, 1992
St Onge S, Chidiac P, Brakier-Gingras L, Bouvier M: β-adrenergic receptor desensitization in the early stage of hereditary cardiomyopathy in hamsters. Can J Physiol Pharmacol 72: 875–883, 1994
Regitz-Zagrosek V, Hertrampf R, Steffen C, Hildebrandt A, Fleck E: Myocardial cyclic AMP and norepinephrine content in human heart failure. Eur Heart J 15(suppl D): 7–13, 1994
Bohm M, La Rosee K, Schwinger RH, Erdmann E: Evidence for reduction of norepinephrine uptake sites in the failing human heart. J Am Coll Cardiol 25: 146–153, 1995
Delehanty JM, Himura Y, Elam H, Hood WB Jr, Liang CS: β-adrenoceptor downregulation in pacing-induced heart failure is associated with increased interstitial NE content. Am J Physiol 266: H930–H935, 1994
Merlet P, Dubois-Rande JL, Adnot S, Bourguignon MH, Benvenuti C, Loisance D, Valette H, Castaigne A, Syrota A: Myocardial β-adrenergic desensitization and neuronal norepinephrine uptake function in idiopathic dilated cardiomyopathy. J Cardiovasc Pharmacol 19: 10–16, 1992
Yoshikawa T, Handa S, Suzuki M, Nagami K: Abnormalities in sympathoneuronal regulation are localized to failing myocardium in rabbit heart. J Am Coll Cardiol 24: 210–215, 1994
Tong J, Ganguly PK, Singal PK: Myocardial adrenergic changes at two stages of heart failure due to adriamycin treatment in rats. Am J Physiol 260: H909–H916, 1991
Liang CS, Frantz RP, Suematsu M, Sakamoto S, Sullebarger JT, Fan TM, Guthinger L: Chronic β-adrenoceptor blockade prevents the development of β-adrenergic subsensitivity in experimental right-sided congestive heart failure in dogs. Circulation 84: 254–266, 1991
Schumacher C, Becker H, Conrads R, Schotten U, Pott S, Kellinghaus M, Sigmund M, Schondube F, Preusse C, Schulte HD: Hypertrophic cardiomyopathy: A desensitized cardiac β-adrenergic system in the presence of normal plasma catecholamine concentrations. Naunyn Schmiedebergs Arch Pharmacol 351: 398–407, 1995
Lu XY, Barnett DB: Differential rates of down regulation and recovery of rat myocardial β-adrenoceptor subtypes in vivo. Eur J Pharmacol 182: 481–486, 1990
Nanoff C, Freissmuth M, Tuisl E, Schutz W: A different desensitization pattern of cardiac β-adrenoceptor subtypes by prolonged in vivo infusion of isoprenaline. J Cardiovasc Pharmacol 13: 198–203, 1989
Vatner DE, Vatner SF, Nejima J, Uemura N, Susanni EE, Hintze TH, Homcy CJ: Chronic norepinephrine elicits desensitization by uncoupling the beta-receptor. J Clin Invest 84: 1741–1748, 1989
Hammond HK, Roth DA, Insel PA, Ford CE, White FC, Maisel AS, Ziegler MG, Bloor CM: Myocardial β-adrenergic receptor expression and signal transduction after chronic volume-overload hypertrophy and circulatory congestion. Circulation 85: 269–280, 1992
Insel PA, Hammond HK: β-adrenergic receptors in heart failure. J Clin Invest 92: 2564, 1993
Bristow MR, Minobe WA, Raynolds MV, Port JD, Rasmussen R, Ray PE, Feldman AM: Reduced β1 receptor messenger RNA abundance in the failing human heart. J Clin Invest 92: 2737–2745, 1993
Engelhardt S, Bohm M, Erdmann E, Lohse MJ: Analysis of β-adrenergic receptor mRNA levels in human ventricular biopsy specimens by quantitative polymerase chain reactions: Progressive reduction of β1-adrenergic receptor mRNA in heart failure. J Am Coll Cardiol 27: 146–154, 1996
Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ: Altered expression of β-adrenergic receptor kinase and β1-adrenergic receptors in the failing human heart. Circulation 87: 454–463, 1993
Urasawa K, Yoshida I, Takagi C, Onozuka H, Mikami T, Kawaguchi H, Kitabatake A: Enhanced expression of β-adrenergic receptor kinase 1 in the hearts of cardiomyopathic Syrian hamsters, BIO53.58. Biochem Biophys Res Commun 219: 26–30, 1996
Ishigai Y, Mori T, Moriyama S, Shibano T: Induction of cardiac β-adrenergic receptor kinase 1 in rat heart failure caused by coronary ligation. J Mol Cell Cardiol 31: 1261–1268, 1999
Anderson KM, Eckhart AD, Willette RN, Koch WJ: The myocardial β-adrenergic system in spontaneously hypertensive heart failure (SHHF) rats. Hypertension 33: 402–407, 1999
Choi DJ, Koch WJ, Hunter JJ, Rockman HA: Mechanism of badrenergic receptor desensitization in cardiac hypertrophy is increased β-adrenergic receptor kinase. J Biol Chem 272: 17223–17229, 1997
Iaccarino G, Dolber PC, Lefkowitz RJ, Koch WJ: β-adrenergic receptor kinase-1 levels in catecholamine-induced myocardial hypertrophy. Hypertension 33: 396–401, 1999
Iaccarino G, Tomhave ED, Lefkowitz RJ, Koch WJ: Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by β-adrenergic receptor stimulation and blockade. Circulation 98: 1783–1789, 1998
Ping P, Gelzer BR, Roth DA, Kiel D, Insel PA, Hammond HK: Reduced β-adrenergic receptor activation decreases G-protein expression and β-adrenergic receptor kinase activity in porcine heart. J Clin Invest 95: 1271–1280, 1995
Cho MC, Rao M, Koch WJ, Thomas SA, Palmiter RD, Rockman HA: Enhanced contractility and decreased β-adrenergic receptor kinase-1 in mice lacking endogenous norepinephrine and epinephrine. Circulation 99: 2702–2707, 1999
Ungerer M, Parruti G, Bohm M, Puzicha M, DeBlasi A, Erdmann E, Lohse MJ: Expression of β-arrestins and β-adrenergic receptor kinases in the failing human heart. Circ Res 74: 206–213, 1994
Bohm M, Moll M, Schmid B, Paul M, Ganten D, Castellano M, Erdmann E: β-adrenergic neuroeffector mechanisms in cardiac hypertrophy of renin transgenic rats. Hypertension 24: 653–662, 1994
Wang J, Liu X, Arneja AS, Dhalla NS: Alterations in protein kinase A and protein kinase C levels in heart failure due to genetic cardiomyopathy. Can J Cardiol 15: 683–690, 1999
Denniss AR, Colucci WS, Allen PD, Marsh JD: Distribution and function of human ventricular β-adrenergic receptors in congestive heart failure. J Mol Cell Cardiol 21: 651–660, 1989
Murphree SS, Saffitz JE: Distribution of β-adrenergic receptors in failing human myocardium. Implications for mechanisms of down-regulation. Circulation 79: 1214–1225, 1989
Lohse MJ: Molecular mechanisms of membrane receptor desensitization. Biochim Biophys Acta 1179: 171–188, 1993
Hammond HK: Mechanisms for myocardial β-adrenergic receptor desensitization in heart failure. Circulation 87: 652–654, 1993
Benovic JL, Bouvier M, Caron MG, Lefkowitz RJ: Regulation of adenylyl cyclase-coupled β-adrenergic receptors. Annu Rev Cell Biol 4: 405–428, 1988
Bohm M, Gierschik P, Knorr A, Larisch K, Weismann K, Erdmann E: Desensitization of adenylate cyclase and increase of Giα in cardiac hypertrophy due to acquired hypertension. Hypertension 20: 103–112, 1992
Marzo KP, Frey MJ, Wilson JR, Liang BT, Manning DR, Lanoce V, Molinoff PB: β-adrenergic receptor-G protein-adenylate cyclase complex in experimental canine congestive heart failure produced by rapid ventricular pacing. Circ Res 69: 1546–1556, 1991
Chen LA, Vatner DE, Vatner SF, Hittinger L, Homcy CJ: Decreased Gsα mRNA levels accompany the fall in Gs and adenylyl cyclase activities in compensated left ventricular hypertrophy. In heart failure, only the impairment in adenylyl cyclase activation progresses. J Clin Invest 87: 293–298, 1991
Longabaugh JP, Vatner DE, Vatner SF, Homcy CJ: Decreased stimulatory guanosine triphosphate binding protein in dogs with pressureoverload left ventricular failure. J Clin Invest 81: 420–424, 1988
Shi B, Heavner JE, McMahon KK, Spallholz JE: Dynamic changes in Gαi-2 levels in rat hearts associated with impaired heart function after myocardial infarction. Am J Physiol 269: H1073–H1079, 1995
Bohm M, Flesch M, Schnabel P: Role of G-proteins in altered badrenergic responsiveness in the failing and hypertrophied myocardium. Basic Res Cardiol 91(suppl 2): 47–51, 1996
Bohm M, Gierschik P, Erdmann E: Quantification of Giα-proteins in the failing and nonfailing human myocardium. Basic Res Cardiol 87(suppl 1): 37–50, 1992
Bohm M, Gierschik P, Knorr A, Schmidt U, Weismann K, Erdmann E: Cardiac adenylyl cyclase, β-adrenergic receptors, and G proteins in salt-sensitive hypertension. Hypertension 22: 715–727, 1993
Bö hm M, Eschenhagen T, Gierschik P, Larisch K, Lensche H, Mende U, Schmitz W, Schnabel P, Scholz H, Steinfath M, Erdmann E: Radioimmunochemical quantification of Giα in right and left ventricles from patients with ischaemic and dilated cardiomyopathy and predominant left ventricular failure. J Mol Cell Cardiol 26: 133–149, 1994
Bohm M, Kirchmayr R, Erdmann E: Myocardial Giα-protein levels in patients with hypertensive cardiac hypertrophy, ischemic heart disease and cardiogenic shock. Cardiovasc Res 30: 611–618, 1995
Feldman AM, Cates AE, Bristow MR, Van DC: Altered expression of α-subunits of G proteins in failing human hearts. J Mol Cell Cardiol 21: 359–365, 1989
Bristow MR, Anderson FL, Port JD, Skerl L, Hershberger RE, Larrabee P, O'Connell JB, Renlund DG, Volkman K, Murray J: Differences in β-adrenergic neuroeffector mechanisms in ischemic vs. idiopathic dilated cardiomyopathy. Circulation 84: 1024–1039, 1991
Neumann J, Schmitz W, Scholz H, von ML, Doring V, Kalmar P: Increase in myocardial Gi-proteins in heart failure. Lancet 2: 936–937, 1988
Feldman AM, Ray PE, Bristow MR: Expression of α-subunits of G proteins in failing human heart: A reappraisal utilizing quantitative polymerase chain reaction. J Mol Cell Cardiol 23: 1355–1358, 1991
Kiuchi K, Shen YT, Vatner SF, Vatner DE: Mechanisms mediating responsiveness to β-adrenergic stimulation after coronary reperfusion in conscious dogs. Am J Physiol 267: H1578–H1588, 1994
Feldman AM, Cates AE, Veazey WB, Hershberger RE, Bristow MR, Baughman KL, Baumgartner WA, Van Dop C: Increase of the 40,000-mol wt pertussis toxin substrate (G protein) in the failing human heart. J Clin Invest 82: 189–197, 1988
Brown LA, Harding SE: The effect of pertussis toxin on β-adrenoceptor responses in isolated cardiac myocytes from noradrenaline-treated guinea-pigs and patients with cardiac failure. Br J Pharmacol 106: 115–122, 1992
Holmer SR, Eschenhagen T, Nose M, Riegger GA: Expression of adenylyl cyclase and G-protein β subunit in end-stage human heart failure. J Cardiac Failure 2: 279–283, 1996
Kawamoto H, Ohyanagi M, Nakamura K, Yamamoto J, Iwasaki T: Increased levels of inhibitory G protein in myocardium with heart failure. Jpn Circ J 58: 913–924, 1994
Bohm M: Alterations of β-adrenoceptor-G-protein-regulated adenylyl cyclase in heart failure. Mol Cell Biochem 147: 147–160, 1995
Fu LX, Bergh CH, Liang QM, Sjogren KG, Xu X, Eriksson P, Hoebeke J, Hjalmarson A: Diabetes-induced changes in the Gi-modulated muscarinic receptor-adenylyl cyclase system in rat myocardium. Pharmacol Toxicol 75: 186–193, 1994
Yamamoto J, Ohyanagi M, Morita M, Iwasaki T: β-adrenoceptor-G protein-adenylate cyclase complex in rat hearts with ischemic heart failure produced by coronary artery ligation. J Mol Cell Cardiol 26: 617–626, 1994
Niroomand F, Bangert M, Beyer T, Rauch B: Reduced adenylyl cyclase inhibition by carbachol and GTP during acute myocardial ischaemia. J Mol Cell Cardiol 24: 471–475, 1992
Katoh Y, Komuro I, Takaku F, Yamaguchi H, Yazaki Y: Messenger RNA levels of guanine nucleotide-binding proteins are reduced in the ventricle of cardiomyopathic hamsters. Circ Res 67: 235–239, 1990
Feldman AM, Tena RG, Kessler PD, Weisman HF, Schulman SP, Blumenthal RS, Jackson DG, Van Dop C: Diminished β-adrenergic receptor responsiveness and cardiac dilation in hearts of myopathic Syrian hamsters (BIO 53.58) are associated with a functional abnormality of the G stimulatory protein. Circulation 81: 1341–1352, 1990
Ransnas LA: The role of G-proteins in transduction of the β-adrenergic response in heart failure. Heart Vessels 6(suppl): 3–5, 1991
Reithmann C, Gierschik P, SD, Werdan K, Jakobs KH: Mechanism of noradrenaline-induced heterologous desensitization of adenylate cyclase stimulation in rat heart muscle cells: Increase in the level of inhibitory G-proteins subunits. Eur J Pharmacol 172: 211–221, 1989
Eschenhagen T, Mende U, Schmitz W, Scholz H, Schulte am EJ, Sempell R, Warnholtz A, Wustel JM: β-adrenoceptor stimulation-induced increase in cardiac Gi-protein expression and in carbachol sensitivity. Eur Heart J 12(suppl F):127–131, 1991
Mende U, Eschenhagen T, Geertz B, Schmitz W, Scholz H, Schulte am Esch J, Sempell R, Steinfath M: Isoprenaline-induced increase in the 40/41 kDa pertussis toxin substrates and functional consequences on contractile response in rat heart. Naunyn Schmiedebergs Arch Pharmacol 345: 44–50, 1992
Eschenhagen T, Mende U, Diederich M, Nose M, Schmitz W, Scholz H, Schulte am EJ, Warnholtz A, Schafer H: Long term β-adrenoceptormediated up-regulation of Giα and Goα mRNA levels and pertussis toxin-sensitive guanine nucleotide-binding proteins in rat heart. Mol Pharmacol 42: 773–783, 1992
Eschenhagen T, Mende U, Nose M, Schmitz W, Scholz H, Schulte am Esch J, Sempell R, Warnholtz A, Wustel JM: Regulation and possible functional implications of G-protein mRNA expression in nonfailing and failing ventricular myocardium. Basic Res Cardiol 87(suppl 1): 51–64, 1992
Eschenhagen T, Mende U, Nose M, Schmitz W, Scholz H, Warnholtz A, Wustel JM: Isoprenaline-induced increase in mRNA levels of inhibitory G-protein-subunits in rat heart. Naunyn Schmiedebergs Arch Pharmacol 343: 609–615, 1991
Ping P, Hammond HK: Diverse G protein and β-adrenergic receptor mRNA expression in normal and failing porcine hearts. Am J Physiol 267: H2079–H2085, 1994
Eschenhagen T, Mende U, Nose M, Schmitz W, Scholz H, Haverich A, Hirt S, Dö ring V, Kalmá r P, Hö ppner W, Seitz H-J: Increased messenger RNA level of the inhibitory G protein a subunit Giα2 in human end-stage heart failure. Circ Res 70: 688–696, 1992
Drazner MH, Peppel KC, Dyer S, Grant AO, Koch WJ, Lefkowitz RJ: Potentiation of β-adrenergic signaling by adenoviral-mediated gene transfer in adult rabbit ventricular myocytes. J Clin Invest 99: 288–296, 1997
Anand-Srivastava MB: G-proteins and adenylyl cyclase signaling in hypertension. Mol Cell Biochem 157: 163–170, 1996
Bouanani N, Corsin A, Gilson N, Crozatier B: β-adrenoceptors and adenylate cyclase activity in hypertrophied and failing rabbit left ventricle. J Mol Cell Cardiol 23: 573–581, 1991
Anand-Srivastava MB: Altered responsiveness of adenylate cyclase to adenosine and other agents in the myocardial sarcolemma and aorta of spontaneously-hypertensive rats. Biochem Pharmacol 37: 3017–3022, 1988
Galinier M, Senard JM, Valet P, Arias A, Daviaud D, Glock Y, Bounhoure JP, Montastruc JL: Cardiac β-adrenoceptors and adenylyl cyclase activity in human left ventricular hypertrophy due to pressure overload. Fundam Clin Pharmacol 8: 90–99, 1994
Ishikawa Y, Sorota S, Kiuchi K, Shannon RP, Komamura K, Katsushika S, Vatner DE, Vatner SF, Homcy CJ: Downregulation of adenylyl cyclase types V and VI mRNA levels in pacing-induced heart failure in dogs. J Clin Invest 93: 2224–2229, 1994
Tanaka R, Fulbright BM, Mukherjee R, Burchell SA, Crawford FA, Zile MR, Spinale FG: The cellular basis for the blunted response to β-adrenergic stimulation in supraventricular tachycardia-induced cardiomyopathy. J Mol Cell Cardiol 25: 1215–1233, 1993
Vatner DE, Kiuchi K, Shannon RP, Vatner SF: Initial changes in badrenergic receptor function during development of rapid ventricular pacing-induced heart failure. In: N.S. Dhalla, P.K. Singal, N. Takeda, R.E. Beamish (eds). Pathophysiology of Heart Failure. 1996, pp 263–276
Sethi R, Dhalla KS, Panagia V, Dhalla NS: Status of post adrenergic receptor mechanisms in cardiac hypertrophy and heart failure. In: N.S. Dhalla, G. Pierce, V. Panagia, R.E. Beamish (eds). Heart Hypertrophy and Failure. 1995, pp 419–446
Sethi R, Dhalla NS: Inotropic responses to isoproterenol in congestive heart failure subsequent to myocardial infarction in rats. J Cardiac Failure 1: 391–399, 1995
Sethi R, Dhalla KS, Beamish RE, Dhalla NS: Differential changes in left and right ventricular adenylyl cyclase activities in congestive heart failure. Am J Physiol 272: H884–H893, 1997
Ganguly PK, Dhalla KS, Shao Q, Beamish RE, Dhalla NS: Differential changes in sympathetic activity in left and right ventricles in congestive heart failure following myocardial infarction. Am Heart J 133: 340–345, 1997
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wang, X., Dhalla, N.S. Modification of β-adrenoceptor signal transduction pathway by genetic manipulation and heart failure. Mol Cell Biochem 214, 131–155 (2000). https://doi.org/10.1023/A:1007131925048
Issue Date:
DOI: https://doi.org/10.1023/A:1007131925048