Abstract
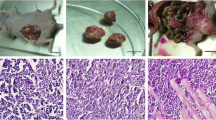
The presence of occult metastasis is the most important factor that influences the prognosis in patients with head and neck cancer. To reproduce occult metastasis of oral cancer cells, we serially resected the primary focus in an orthotopic implantation model to examine when metastasis of cancer cells occurs. Human squamous cell carcinoma was implanted into the tongue of nude mice divided into two groups, non-surgery and surgery groups. Mice in the non-surgery group were sacrificed, and the tongue cancer and cervical lymph nodes were resected simultaneously. In the surgery-group, resection of the tongue cancer was performed, and the cervical lymph nodes were resected on day 28. For the non-surgery-group, the incidences of metastasis were 0%, 9%, 36%, 91% and 100% on days 3, 7, 14, 21 and 28, respectively. For the surgery-group, resection of the tongue cancer was performed on days 3, 7 and 14, and the incidence of metastasis on day 28 was 0%, 82% and 91%, respectively. The occult metastasis was reproduced using resected primary cancer on day 7. This time-based model may be useful to clarify the mechanism of metastasis and to develop new treatments.
Similar content being viewed by others
References
Shah JP, Lydiatt W. Treatment of cancer of the head and neck. CA Cancer J Clin 1995; 45: 352–68.
Kowalski LP, Magrin J, Waksman G, Santo GF et al. Supraomohyoid neck dissection in the treatment of head and neck tumors. Survival results in 212 cases. Arch Otolaryngol Head Neck Surg 1993; 119: 958–63.
Martinez-Gimeno C, Rodriguez EM, Vila CN, Varela CL. Squamous cell carcinoma of the oral cavity: a clinicopathologic scoring system for evaluating risk of cervical lymph node metastasis. Laryngoscope 1995; 105: 728–33.
Close LG, Merkel M, Vuitch MF et al. Computed tomograghic evaluation of regional lymph node involvement in cancer of the oral cavity and oropharynx. Head Neck 1989; 11: 309–17.
Lefebvre JL, Coche-Dequeant B, Buisset E et al. Management of early oral cavity cancer. Experience of centre oscar lambret. Oral Oncol, Eur J Cancer 1994; 30B: 216–20.
Kawashiri S, Kumagai S, Kojima K et al. Development of a new invasion and metastasis model of human oral squamous cell carcinomas. Oral Oncol, Eur J Cancer 1995; 31B: 216–21.
Yokoi T, Yamaguchi A, Odajima T, Furukawa K. Establishment and characterization of a human cell line derived from a squamous cell carcinoma of the tongue. Tumor Res 1988; 23: 43–57.
Kawashiri S, Kumagai S, Kojima K et al. Experimental studies on the in vivo model for invasion and metastasis of oral squamous cell carcinoma. Part 1: establishment of orthotopic implantation model and investigation of invasive activity. In Varma AK, Mori M (eds): Oral Oncology, 4B. India: Macmillan 1995; 279–82.
Moll R, Frnake WW, Schiller DL et al. The catalog of human cytokeratins pattern of expression in normal epithelia, tumor and cultured cell. Cell 1982; 31: 11–24.
Liotta LA, Wewer U, Rao NC et al. Biochemical mechanisms of tumor invasion and metastasis. Adv Exp Med Biol 1988; 233: 161–9.
Fidler IJ. Selection of successive tumor lines for metastasis. Nature New Biol 1973; 242: 148–9.
Maehara Y, Oshiro T, Endo K et al. Clinical significance of occult micrometastasis lymph nodes from patients with early gastric cancer who died of recurrence. Surgery 1996; 119: 397–402.
van den Brekel MW, Castelijns JA, Stel HV et al. Occult metastatic neck disease: detection with US and US-guided fine-needle aspiration cytology. Radiology 1991; 180: 457–61.
Sancho-Garnier H, Richard JM, Micheau C et al. Prognostic factors in cervical lymph node metastasis in upper respiratory and digestive tract carcinomas: study of 1713 cases during a 15-year period. Laryngoscope 1987; 97: 97–101.
Nishijima W, Takooda S, Usui H, Negishi T. Simultaneous bilateral neck dissections. Nippon Jibiinkoka Gakkai Kaiho 1991; 94: 1104–12.
van den Hoogen FJ, Manni JJ. Value of the supraomohyoid neck dissection with frozen section analysis as a staging procedure in the clinically negative neck in squamous cell carcinoma of the oral cavity. Eur Arch Otorhinolaryngol 1992; 249: 144-8.
Alvi A, Johnson JT. Extracapsular spread in the clinically negative neck (N0): implications and outcome. Otolaryngol Head Neck Surgy 1996; 114: 65–70.
Jones AS, Phillips DE, Helliwell TR, Roland NJ. Occult node metastases in head and neck squamous carcinoma. Eur Arch Otorhinolaryngol 1993; 250: 446–9.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kawashiri, S., Kumagai, S., Kojima, K. et al. Reproduction of occult metastasis of head and neck cancer in nude mice. Clin Exp Metastasis 17, 277–282 (1999). https://doi.org/10.1023/A:1006618332558
Issue Date:
DOI: https://doi.org/10.1023/A:1006618332558