Abstract
Purpose. The aim of the study was to investigate the effect of histidine on the stability and physical properties of a fully human anti-IL8 monoclonal antibody (ABX-IL8) in aqueous and solid forms.
Methods. Using a fractional factorial design, we tested many excipients, including histidine, sucrose, and other commonly used excipients, on the stability and physical properties of the antibody in both liquid and lyophilized forms. Antibody stability and physical properties were evaluated using size-exclusion high-performance liquid chromatography (SEC-HPLC), sodium dodecyl sulfate-polyacryla- mide gel electrophoresis (SDS-PAGE), and a viscometer. Residual moisture content was determined by coulometric Karl Fischer titrator. Differential scanning calorimetry (DSC) was used to detect the glass transition temperatures (T g) of the solid cakes and melting temperatures (T m) of the antibody in liquid formulations. Fourier-transform infrared (FTIR) spectroscopy was used to examine the overall secondary structure.
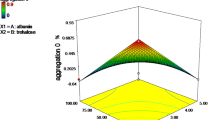
Results. Increasing the histidine concentration in the bulk solution inhibited the increases of high-molecular-weight (HMW) species and aggregates upon lyophilization and storage. In addition, histidine bulk enhanced solution stability of the antibody under freezing and thermal stress conditions, as evidenced by the lower levels of aggregates. Furthermore, histidine reduced viscosity of the antibody solution, which is desirable for the manufacture of the dosage form. However, high concentrations of histidine in liquid formulations led to coloration of the solution and high levels of aggregates on storage at elevated temperature (40°C) after the formulations were exposed to stainless steel containers during bulk freezing-thawing.
Conclusions. Histidine enhanced the stability of ABX-IL8 in both aqueous and lyophilized forms. Histidine also improved the physical properties such as reducing the solution viscosity. Liquid formulations containing high concentrations of histidine should not be stored in stainless steel tanks at elevated temperatures.
Similar content being viewed by others
REFERENCES
T. Osterberg, A. Fatouros, and M. Mikaelsson. Development of freeze-dried albumin-free formulation of recombinant factor VIII SQ. Pharm. Res. 14:892-898 (1997).
J. L. Cleland, X. Lam, B. Kendrick, J. Yang, T. H. Yang, D. Overcashier, D. Brooks, C. Hsu, and J. F. Carpenter. A specific molar ratio of stabilizer to protein is required for storage stability of a lyophilized monoclonal antibody. J. Pharm. Sci. 90:310-321 (2001).
J. C. Lee and S. N. Timasheff. The stabilization of proteins by sucrose. J. Biol. Chem. 256:7193-7201 (1981).
B. S. Kendrick, B. S. Chang, T. Arakawa, B. Peterson, T. W. Randolph, M. C. Manning, and J. F. Carpenter. Preferential exclusion of sucrose from recombinant interleukin-1 receptor antagonist: role in restricted conformational mobility and compaction of native state. Proc. Natl. Acad. Sci. USA 94:11917-11922 (1997).
R. A. DePaz, C. C. Barnett, D. A. Dale, J. F. Carpenter, A. L. Gaertner, and T. W. Randolph. The excluding effects of sucrose on a protein chemical degradation pathway: methionine oxidation in subtilisin. Arch. Biochem. Biophys. 384:123-132 (2000).
L. L. Green. Antibody engineering via genetic engineering of the mouse: XenoMouse strains are a vehicle for the facile generation of therapeutic human monoclonal antibodies. J. Immunol. Methods 231:11-23 (1999).
X. D. Yang, J. R. Corvalan, P. Wang, C. M. Roy, and C. G. Davis. Fully human anti-interleukin-8 monoclonal antibodies: potential therapeutics for the treatment of inflammatory disease states. J. Leukoc. Biol. 66:401-410 (1999).
W. J. Youden and E. H. Steiner. Statistical Manual of the Association of Official Analytical Chemists, AOAC International, Arlington, VA, pp. 51-58 (1987).
J. C. May, E. Grim, R. M. Wheeler, and J. West. Determination of residual moisture in freeze-dried viral vaccines: Karl Fischer gravimetric and thermogravimetric methodologies. J. Biol. Stand. 10:249-259 (1982).
E. P. A. Manual. 200.7. Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry, U.S. Environmental Protection Agency, Cincinnati (1994).
M. J. Pikal, K. M. Dellerman, M. L. Roy, and R. M. Riggin. The effects of formulation variables on the stability of freeze-dried human growth hormone. Pharm. Res. 8:427-436 (1991).
R. L. RemmeleJr., N. S. Nightlinger, S. Srinivasan, and W. R. Gombotz. Interleukin-1 receptor (IL-1R) liquid formulation development using differential scanning calorimetry. Pharm. Res. 15:200-208 (1998).
L. Kreilgaard, S. Frokjaer, J. M. Flink, T. W. Randolph, and J. F. Carpenter. Effects of additives on the stability of recombinant human factor XIII during freeze-drying and storage in the dried solid. Arch. Biochem. Biophys. 360:121-134 (1998).
E. D. Breen, J. G. Curley, D. E. Overcashier, C. C. Hsu, and S. J. Shire. Effect of moisture on the stability of a lyophilized humanized monoclonal antibody formulation. Pharm. Res. 18:1345-1353 (2001).
F. Franks, R. H. M. Hatley, and S. F. Mathias. Material science and the production of shelf stable biologicals. BioPharm 4:38-55 (1991).
M. J. Pikal. Mechanisms of Protein Stabilization During Freeze-Drying and Storage: the Relative Importance of Thermodynamic Stabilization and Glassy State Relaxation Dynamics, Freeze-drying/lyophilization of pharmaceutical and biological products. Drugs and the pharmaceutical sciences. Volume 96. Marcel Dekker, New York (1999).
R. H. Hatley. Glass fragility and the stability of pharmaceutical preparations—excipient selection. Pharm. Dev. Technol. 2:257-264 (1997).
B. Chen, H. R. Costantino, J. Liu, C. C. Hsu, and S. J. Shire. Influence of calcium ions on the structure and stability of recombinant human deoxyribonuclease I in the aqueous and lyophilized states. J. Pharm. Sci. 88:477-482 (1999).
A. Dong, S. J. Prestrelski, S. D. Allison, and J. F. Carpenter. Infrared spectroscopic studies of lyophilization-and temperature-induced protein aggregation. J. Pharm. Sci. 84:415-424 (1995).
S. D. Allison, T. W. Randolph, M. C. Manning, K. Middleton, A. Davis, and J. F. Carpenter. Effects of drying methods and additives on structure and function of actin: mechanisms of dehydration-induced damage and its inhibition. Arch. Biochem. Biophys. 358:171-181 (1998).
J. F. Carpenter, S. J. Prestrelski, and T. Arakawa. Separation of freezing-and drying-induced denaturation of lyophilized proteins using stress-specific stabilization. I. Enzyme activity and calorimetric studies. Arch. Biochem. Biophys. 303:456-464 (1993).
R. L. RemmeleJr., C. Stushnoff, and J. F. Carpenter. Real-time in situ monitoring of lysozyme during lyophilization using infrared spectroscopy: dehydration stress in the presence of sucrose. Pharm. Res. 14:1548-1555 (1997).
S. D. Allison, A. Dong, and J. F. Carpenter. Counteracting effects of thiocyanate and sucrose on chymotrypsinogen secondary structure and aggregation during freezing, drying, and rehydration. Biophys. J. 71:2022-2032 (1996).
G. Xie and S. N. Timasheff. The thermodynamic mechanism of protein stabilization by trehalose. Biophys. Chem. 64:25-43 (1997).
T. Arakawa, S. J. Prestrelski, W. C. Kenney, and J. F. Carpenter. Factors affecting short-term and long-term stabilities of proteins. Adv. Drug Deliv. Rev. 46:307-326 (2001).
J. F. Carpenter and J. H. Crowe. The mechanism of cryoprotection of proteins by solutes. Cryobiology 25:244-255 (1988).
S. D. Allison, B. Chang, T. W. Randolph, and J. F. Carpenter. Hydrogen bonding between sugar and protein is responsible for inhibition of dehydration-induced protein unfolding. Arch. Biochem. Biophys. 365:289-298 (1999).
T. Arakawa, Y. Kita, and J. F. Carpenter. Protein-solvent interactions in pharmaceutical formulations. Pharm. Res. 8:285-291 (1991).
B. S. Chang, R. M. Beauvais, T. Arakawa, L. O. Narhi, A. Dong, D. I. Aparisio, and J. F. Carpenter. Formation of an active dimer during storage of interleukin-1 receptor antagonist in aqueous solution. Biophys. J. 71:3399-3406 (1996).
A. Fatouros, T. Osterberg, and M. Mikaelsson. Recombinant factor VIII SQ—inactivation kinetics in aqueous solution and the influence of disaccharides and sugar alcohols. Pharm. Res. 14:1679-1684 (1997).
R. I. Senderoff, K. M. Kontor, L. Kreilgaard, J. J. Chang, S. Patel, J. Krakover, J. K. Heffernan, L. B. Snell, and G. B. Rosenberg. Consideration of conformational transitions and racemization during process development of recombinant glucagon-like peptide-1. J. Pharm. Sci. 87:183-189 (1998).
J. Liu, E. D. Breen, B. Chen, and S. J. Shire. Investigation of thermodynamic properties and stability of a highly concentrated antibody in aqueous solution. American Association of Pharmaceutical Scientists (AAPS) Annual Meeting, Indianapolis, Indiana, 2000.
P. M. Tessier, H. R. Johnson, R. Pazhianur, B. W. Berger, J. L. Prentice, B. J. Bahnson, S. I. Sandler, and A. M. Lenhoff. Predictive crystallization of ribonuclease A via rapid screening of osmotic second virial coefficients. Proteins 50:303-311 (2003).
J. C. Wataha, S. K. Nelson, and P. E. Lockwood. Elemental release from dental casting alloys into biological media with and without protein. Dent. Mater. 17:409-414 (2001).
X. M. Lam, J. Y. Yang, and J. L. Cleland. Antioxidants for prevention of methionine oxidation in recombinant monoclonal antibody HER2. J. Pharm. Sci. 86:1250-1255 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, B., Bautista, R., Yu, K. et al. Influence of Histidine on the Stability and Physical Properties of a Fully Human Antibody in Aqueous and Solid Forms. Pharm Res 20, 1952–1960 (2003). https://doi.org/10.1023/B:PHAM.0000008042.15988.c0
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000008042.15988.c0