Abstract
Purpose. To evaluate the in vitro and in vivo characteristics of hypromellose (HPMC) capsules prepared using a gellan gum and potassium gelling system compared to conventional hard gelatin capsules.
Methods. The in vitro dissolution of ibuprofen gelatin and HPMC capsules was determined using the USP and TRIS buffers at pH 7.2. The effect of pH and composition of the media was determined using a model drug that is soluble throughout the pH range 1.2 to 7.2. In an 11 subject four-way crossover study, the gastrointestinal performance of ibuprofen gelatin and HPMC capsule formulations was evaluated using scintigraphy and pharmacokinetics following fasted and fed dosing.
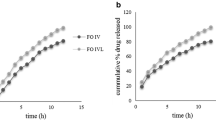
Results. Acid conditions and the presence of K+ cations hinder HPMC capsule opening, whereas in water, dissolution is identical to that of gelatin. These effects are related to the nature of the gel network that is formed in the presence of cations. No significant difference in esophageal transit was observed. Although the in vivo opening times of HPMC capsules were longer than for their gelatin counterparts, no significant difference in the regulatory important pharmacokinetic metrics of Cmax and AUC was found between ibuprofen, gelatin and HPMC capsules.
Conclusions. The in vitro performance of HPMC capsules differ from gelatin, which will require modification to dissolution testing methodology for certain drugs. However, for the class II BCS drug ibuprofen, the two capsule types were not statistically different when comparing AUC and Cmax values, which suggests that the in vitro differences have reduced in vivo relevance.
Similar content being viewed by others
references
R. F. T. Stepto and I. Tomka. Injection molding of natural hydrophilic polymers in the presence of water. Chimia 41:76–81 (1987).
T. Ogura, Y. Furuya, and S. Matsuura. HPMC capsules — An alternative to gelatin. Pharm. Tech. Eur. 11:32–42 (1998).
S. Nagata and B. E. Jones. Hard two-piece capsules and the control of drug delivery. Eur. Pharm. Rev. 5:41–46 (2000).
O. Honkanen, H. Seppä, S. Eerikäinen, R. Tuominen, and M. Marvola. Bioavailability of Ibuprofen from orally and rectally administered hydroxypropyl methyl cellulose capsules compared to corresponding gelatin capsules. STP Pharma Sciences 11:181–185 (2001).
G. K. Greminger Jr. and L. E. Davis. Preparation of medicinal capsule shells from hydroxyalkyl-alkyl cellulose ethers. UK Patent No. 1 144 225 (1969).
R. R. Grosswald, J. B. Anderson, and C. S. Andrew. Method for the manufacture of pharmaceutical cellulose capsules. US Patent No. 5 698 155 (1997).
T. Yamamoto, K. Abe, and S. Matsuura. Hard capsule for pharmaceutical drugs and method for producing the same. US Patent No. 5 264 223(1993).
D. Cade, X. He, and R. A. Scott. Polymer film compositions for capsules. European Patent No. 0 946 637 B1 (2001).
H. Grasdalen and O. Smidsrød. Gelation of gellan gum. Carbohydr. Polym. 7:371–393 (1987).
M. Watase and K. Nishinari. Thermal and rheological properties of kappa-carrageenan gels containing alkali earth metal ions. In G. O. Phillips, D. J. Wedlock, and P. A. Williams (eds.), Gums and Stabilisers for the Food Industry 3, Elsevier, London, 1986 pp. 185–194.
J. Doyle, P. Giannouli, K. Philp, and E. R. Morris. Effect of K+ and Ca2+ cations on gelation of ϰ-carrageenan. In G. O. Phillips and P. A. Williams (eds.), Gums and Stabilisers for the Food Industry 11, Royal Society of Chemistry, Cambridge, UK, 2002 pp. 158–164.
G. H. Therkelsen. Carrageenan. In R. L. Whistler and J. N. BeMiller (eds.), Industrial Gums, Polysaccharides and their Derivatives, Academic Press, New York, 1993 pp. 145–180.
K. S. Kang and D. J. Pettitt. Xanthan, Gellan, Welan and Rhamsan. In R. L. Whistler and J. N. BeMiller (eds.), Industrial Gums, Polysaccharides and their Derivatives, Academic Press, New York, 1993 pp. 341–397.
G. R. Sanderson and R. C. Clark. Gellan gum, a new gelling polysaccharide. In G. O. Phillips, D. J. Wedlock, and P. A. Williams (eds.), Gums and Stabilisers for the Food Industry 2, Pergamon Press, Oxford, 1984, pp. 201–210.
T. Turquois, C. Rochas, and F. R. Taravel. Rheological studies of synergistic kappa carrageenan-carob galactomannan gels. Carbohydr. Polym. 17:263–268 (1992).
L. Piculell. Gelling carrageenans. In A. M. Stephen (ed.), Food Polysaccharides and their Applications, Marcel Dekker, New York, 1995 pp. 205–244.
S. Tochio, S. Nagata, and S. Yamashita. The influence of the composition of the test fluids on dissolution from HPMC capsules. AAPS Pharm. Sci., 4(4): Abstract W4340 (2002).
D. F. Salo, R. Lavery, V. Varma, J. Goldberg, T. Shapiro, and A. A. Kenwood. Randomized, clinical trial comparing oral celecoxib 200 mg, celecoxib 400 mg, and ibuprofen 600 mg for acute pain. Acad. Emerg. Med. 10:22–30 (2003).
I. R. Wilding, S. S. Davis, K. P. Steed, R. A. Sparrow, J. Westrup, and J. M. Hempenstall. Gastrointestinal transit of a drug-resinate administered as an oral suspension. Int. J. Pharm. 101:263–268 (1994).
M. Frier and A. C. Perkins. Radiopharmaceuticals and the gastrointestinal tract. Eur. J. Nucl. Med. 21:1234–1242 (1994).
A. L. Connor, H. A. Wray, I. R. Wilding, R. J. Dansereau, D. Wenderoth, S. Hathaway, Z Li. The upper gastrointestinal transit of the film-coated risedronate tablet is independent of the volume of water ingested. Practical Gastroenterology 25:26, 31, 35, 37, 40, 41, 45, 46 (2001).
O. Honkanen, P. Laaksonen, J. Marvola, S. Eerikáinen, R. Tuominen, and M. Marvola. Bioavailability and in vitro oesophageal sticking tendency of hydroxypropyl methylcellulose capsule formulations and corresponding gelatin capsule formulations. Eur. J. Pharm. Sci. 15:479–488 (2002).
G. Ponchel and G. Degobert. Hydroxypropyl methylcellulose hard capsules: alternative to gelatin—comparative study of their adhesiveness. STP Pharma. Pratiques. 9:38–43 (1999).
E. R. Morris, M. G. E. Gothard, M. W. N. Hember, C. E. Manning, and G. Robinson. Conformational and rheological transitions of welan, rhamsan, and acylated gellan. Carbohydr. Polym. 30:165–175 (1996).
J. Brown, N. Madit, E. T. Cole, I. R. Wilding, and D. Cadé. The effect of cross-linking on the in vivo disintegration of hard gelatin capsules. Pharm. Res. 15:1026–1030 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cole, E.T., Scott, R.A., Cade, D. et al. In Vitro and in Vivo Pharmacoscintigraphic Evaluation of Ibuprofen Hypromellose and Gelatin Capsules. Pharm Res 21, 793–798 (2004). https://doi.org/10.1023/B:PHAM.0000026430.73789.e6
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000026430.73789.e6