Abstract
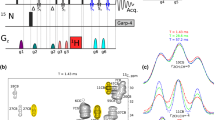
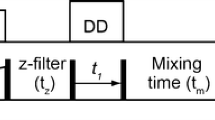
A CC(CO)NH TOCSY-based 3D pulse scheme is presented for measuring 1H-13C dipole-dipole cross-correlated relaxation at CH2 positions in uniformly 13C-, 15N-labeled proteins. Simulations based on magnetization evolution under relaxation and scalar coupling interactions show that cross-correlation rates between 1H-13C dipoles in CH2 groups can be simply obtained from the intensities of 13C triplets. The normalized cross-correlation relaxation rates are related to cross-correlation order parameters for macromolecules undergoing isotropic motion, which reflect the degrees of spatial restriction of CH2 groups. The study on human intestinal fatty acid binding protein (131 residues) in the presence of oleic acid demonstrates that side chain dynamics at most CH2 positions can be characterized for proteins less than 15 kDa in size, with the proposed TOCSY-based approach.
Similar content being viewed by others
References
Bremi, T. and Bruschweiler, R. (1997) J. Am. Chem. Soc., 119, 6672–6673.
Brutscher, B., Bruschweiler, R. and Ernst, R.R. (1997) Biochemistry, 36, 13043–13053.
Daragan, V.A. and Mayo, K.H. (1995) J. Magn. Reson. Ser. B, 107, 274–278.
Daragan, V.A. and Mayo, K.H. (1997) Prog. NMR Spectrosc., 31, 63–105.
Daragan, V.A., Kloczewiak, M.A. and Mayo, K.H. (1993) Biochemistry, 32, 10580–10590.
Delaglio, F., Grzesiek, S., Vuister, G.W., Zhu, G., Pfeifer, J. and Bax, A. (1995) J. Biomol. NMR, 6, 277–293.
Eisenmesser, E.Z., Bosco, D.A., Akke, M. and Kern, D. (2002) Science, 295, 1520–1523.
Emsley, L. and Bodenhausen, G. (1992) J. Magn. Reson., 97, 135.
Engelke, J. and Rüterjans, H. (1998) J. Biomol. NMR, 11, 165–183.
Ernst, M. and Ernst, R.R. (1994) J. Magn. Reson. Ser. A, 110, 202–213.
Fischer, M.W.F., Zeng, L., Pang, Y., Hu, W., Majumdar, A. and Zuiderweg, E.R.P. (1997) J. Am. Chem. Soc., 119, 12629–12642.
Frueh, D. (2002) Prog. NMR Spectrosc., 41, 305–324.
Garrett, D.S., Powers, R., Gronenborn, A.M. and Clore, G.M. (1991) J. Magn. Reson., 95, 214–220.
Ghose, R. and Prestegard, J.H. (1998) J. Magn. Reson., 134, 308–314.
Ghose, R., Huang, K. and Prestegard, J.H. (1998) J. Magn. Reson., 135, 487–499.
Kay, L.E. (1998) Biochem. Cell Biol., 76, 145–52.
Kay, L.E. and Bull, T.E. (1992) J. Magn. Reson., 99, 615–622.
Kay, L.E., Keifer, P. and Saarinen, T. (1992) J. Am. Chem. Soc., 114, 10663–10665.
Korzhnev, D.M., Billeter, M., Arseniev, A.S. and Orekhov, V.Y. (2001) Prog. NMR Spectrosc., 38, 197–266.
LeMaster, D.M. and Kushlan, D.M. (1996) J. Am. Chem. Soc., 118, 9255–9264.
Lipari, G. and Szabo, A. (1982) J. Am. Chem. Soc., 104, 4546–4559.
Liu, W.D., Zheng, Y., Cistola, D.P. and Yang, D.W., J. Biomol. NMR, in press.
London, R.E. and Avitabile, J. (1978) J. Am. Chem. Soc., 100, 7159–7165.
Mayne, C.L., Grant, D.M. and Alderman, D.W. (1976) J. Chem. Phys., 65, 1684–1695.
Millet, O., Muhandiram, D.R., Skrynnikov, N.R. and Kay, L.E. (2002) J. Am. Chem. Soc., 124, 6439–6448.
Montelione, G.T., Lyons, B.A., Emerson, S.D. and Tashiro, M. (1992) J. Am. Chem. Soc., 114, 10974–10975.
Muhandiram, D.R., Yamazaki, T., Sykes, B.D. and Kay, L.E. (1995) J. Am. Chem. Soc., 117, 11536–11544.
Nesmelova, I., Krushelnitsky, A., Idiyatullin, D., Blanco, F., Ramirez-Alvarado, M., Daragan, V.A., Serrano, L. and Mayo, K.H. (2001) Biochemistry, 40, 2844–2853.
Palmer, A.G. (2001) Annu. Rev. Biophys. Biomol. Struct., 30, 129–155.
Palmer, A.G., Williams, J. and McDermott, A. (1996) J. Phys. Chem., 100, 13293–13310.
Pervushin, K., Riek, R., Wider, G. and Wüthrich, K. (1997) Proc Natl. Acad. Sci. USA, 94, 12366–12371.
Prestegard, J.H. and Grant, D.M. (1978) J. Am. Chem. Soc., 100, 4664–4668.
Redfield, A.G. (1957) IBM J. Res. Dev., 1, 19.
Reif, B., Hennig, M. and Griesinger, C. (1997) Science, 276, 1230–1233.
Tjandra, N., Szabo, A. and Bax, A. (1996) J. Am. Chem. Soc., 118, 6986–6991.
Wand, A.J. (2001) Nat. Struct. Biol., 8, 926–931.
Werbelow, L.G. and Grant, D.M. (1975) J. Chem. Phys., 63, 544–557.
Yang, D.W., Konrat, R. and Kay, L.E. (1997) J. Am. Chem. Soc., 119, 11938–11940.
Yang, D.W., Mittermaier, A., Mok, Y.K. and Kay, L.E. (1998a) J. Mol. Biol., 276, 939–954.
Yang, D.W., Tolman, J.R., Goto, N.K. and Kay, L.E. (1998b) J. Biomol. NMR, 12, 325–332.
Ye, C., Fu, R., Hu, J., Hou, L. and Ding, S. (1993) Magn. Reson. Chem., 31, 699–704.
Zhang, F.L., Lucke, C., Baier, L.J., Sacchettini, J.C. and Hamilton, J.A. (1997) J. Biomol. NMR, 9, 213–228.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zheng, Y., Yang, D. Measurement of Dipolar Cross-Correlation in Methylene Groups in Uniformly 13C-, 15N-Labeled Proteins. J Biomol NMR 28, 103–116 (2004). https://doi.org/10.1023/B:JNMR.0000013826.82936.0e
Issue Date:
DOI: https://doi.org/10.1023/B:JNMR.0000013826.82936.0e