Abstract
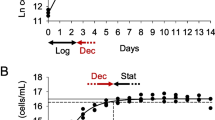
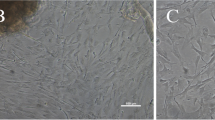
Gill epithelia from freshwater rainbow trout can be grown in primary culture in Leibovitz's L-15 medium, by seeding freshly isolated gill cells on two successive days, from two different fish, directly onto permeable filter supports (DSI technique). This preparation allows the measurement of transepithelial resistance (TER) and exposure of the apical surface to freshwater, as in vivo. New culture methods were developed and evaluated, using TER as an indicator of epithelial integrity, in an effort to improve the utility of the preparation for proteomic and toxicological research. TER was not related to cell density or protein content in DSI epithelia. To eliminate bovine proteins, the 5% foetal bovine serum (FBS) normally required for epithelial development was replaced with trout plasma. While previously frozen trout plasma proved toxic, freshly collected heparinized plasma, provided by chronically cannulated adult trout, was not. The use of 5% fresh trout plasma supported a TER development curve identical to that with 5% FBS, a useful advance for proteomic research because foreign (bovine) proteins are eliminated. However, 10% plasma reduced TER development, and 100% plasma abolished it. The inhibitory effect on TER of high plasma levels was seen only early in epithelial development, and was exerted from the apical side, likely an effect on tight junction formation. Mature plasma-supplemented preparations mounted a TER rise in response to apical freshwater exposure comparable to that of FBS-supplemented epithelia. Yolk-sac fry extract was inhibitory to TER development, even in the presence of 5% FBS. Transfer of mature epithelia from 18 °C to 4 °C maintained stable TER and extended the useable lifespan by at least ten days, thereby facilitating storage of preparations for toxicity testing. A new method of growing epithelia, involving only a single seeding of cells from a single fish, directly onto filter inserts (SDSI technique), provided mature epithelia with much lower TER, a smaller TER response to apical freshwater, and lower cell density and protein content than DSI epithelia. These SDSI epithelia offer the advantage of multiple preparations grown directly from unique individuals for in vitro toxicity testing.
Similar content being viewed by others
References
Alexander, J.B. and Ingram, G.A. 1980. A comparison of five of the methods commonly used to measure protein concentrations in fish sera. J. Fish. Biol. 16: 115–122.
Avella, M. and Ehrenfeld, J. 1997. Fish gill respiratory cells in culture: a new model for Cl–-secreting epithelia. J. Membrane Biol. 156: 87–97.
Aziz, A.B., Chorin, M., Monselise, S.P. and Reichert, I. 1962. Established eurythermic line of fish cells in vitro. Science 135: 1065–1067.
Bols, N.C., Boliska, S.A., Dixon, D.G., Hodson, P.V. and Kaiser, K.L.E. 1985. The use of fish cell cultures as an indication of contaminant toxicity to fish. Aquat. Toxicol. 6: 147–155.
Bols, N.C., Mosser, D.D. and Steels, G.B. 1992. Temperature studies and recent advances with fish cells in vitro. Comp. Biochem. Physiol. 103A: 1–14.
Bradford, C.S., Sun, L., Collodi, P. and Barnes, D.W. 2000. Cell cultures from zebrafish embryos and adult tissues. Methods Cell Sci. 22: 99–107.
Butt, A., Davison, M.D., Smith, G.J., Young, J.A., Gaskell, S.J., Oliver, S.G. and Beynon, R.J. 2001. Chromatographic separations as a prelude to two-dimensional electrophoresis in proteomics analysis. Proteomics 1: 42–53.
Carlsson, C. and Pärt, P. 2001. 7–ethoxyresorufin O-deethylase induction in rainbow trout gill epithelium cultured on permeable supports: asymmetrical distribution of substrate metabolites. Aquat. Toxicol. 54: 29–38.
Cereijido, M., Meza, I. and Martínez-Palomo, A. 1981. Occluding junctions in cultured epithelial monolayers. Am. J. Physiol. 240: C96–C102.
Chang, C.-W.,Wang, X. and Caldwell, R. 1997a. Serum opens tight junctions and reduces ZO-1 protein in retinal epithelial cells. J. Neurochem. 69: 859–867.
Chang, C.-W., Ye, L., Defoe, D. and Caldwell, R.B. 1997b. Serum inhibits tight junction formation in cultured pigment epithelial cells. Investig. Ophthalmol. Visual Sci. 38: 1082–1093.
Collodi, P. and Barnes, D.W. 1990. Mitogenic activity from trout embryos. Proc. Natl. Acad. Sci. USA 87: 3498–3502.
Conyers, G., Milks, L., Conklyn, M., Showell, H. and Cramer, E. 1990. A factor in serum lowers resistance and opens tight junctions of MDCK cells. Am. J. Physiol. 259: C577–C585.
Dotto G.P. 1999. Signal transduction pathways controlling the switch between keratinocyte growth and differentiation. Crit. Rev. Oral Biol. Med. 10: 442–457.
Faurskov, B. and Bjerregaard, H.F. 1999. Effects of cisplatin on transepithelial resistance and ion transport in the A6 renal epithelial cell line. Toxicol. In Vitro 13: 611–617.
Fletcher M. 1997. Electrophysiological and ion transport characteristics of cultured branchial epithelia from freshwater rainbow trout. M.Sc. Thesis, Dept. of Biology, McMaster University, Hamilton, Canada. 174 pp.
Fletcher, M., Kelly, S., Pärt, P., O'Donnell, M.J. and Wood, C.M. 2000. Transport properties of cultured branchial epithelia from freshwater rainbow trout: a novel preparation with mitochondriarich cells. J. Exp. Biol. 203: 1523–1537.
Fryer, J.L., Yusha, A. and Pilcher, K.S. 1965. The in vitro cultivation of tissue and cells of Pacific salmon and steelhead trout. Ann. N.Y. Acad. Sci. 126: 566–586.
Gilmour, K., Fletcher, M. and Pärt, P. 1998a. Transepithelial potential of cultured branchial epithelia from rainbow trout under symmetrical conditions. In Vitro Cell. Dev. Biol. Anim. 34: 436–438.
Gilmour, K.M., Pärt, P., Prunet, P., Pisam, M., McDonald, D.G. and Wood, C.M. 1998b. Permeability and morphology of a cultured branchial epithelium from the rainbow trout during prolonged apical exposure to freshwater. J. Exp. Zool. 281: 531–545.
Hansen, H.J.M., Kelly, S.P., Grosell, M. and Wood, C.M. 2002. Studies on lipid metabolism in trout (Oncorhynchus mykiss) branchial cultures. J. Exp. Zool. 293: 683–692.
Hightower, L. and Renfro, J.L. 1988. Recent applications of fish cell culture to biomedical research. J. Exp. Zool. 248: 290–302.
Kelly, S.P., Fletcher, M., Pärt, P. and Wood, C.M. 2000. Procedures for the preparation and culture of ‘reconstructed’ rainbow trout branchial epithelia. Methods Cell Sci. 22: 153–163.
Kelly, S.P. and Wood, C.M. 2001a. The cultured branchial epithelium of the rainbow trout as a model for diffusive fluxes of ammonia across the fish gill. J. Exp. Biol. 204: 4115–4124.
Kelly, S.P. and Wood, C.M. 2001b. Effect of cortisol on the physiological properties of a cultured pavement cell epithelium from freshwater rainbow trout gills. Am. J. Physiol. 281: R811–R820.
Kelly, S.P. and Wood, C.M. 2002a. Prolactin effects on cultured pavement cell epithelia and pavement cell plus mitochondria-rich cell epithelia from freshwater rainbow trout gills. Gen. Comp. Endocrinol. 128: 44–56.
Kelly, S.P. and Wood, C.M. 2002b. Cultured gill epithelia from freshwater tilapia (Oreochromis niloticus): Effect of cortisol and homologous serum supplements from stressed and unstressed fish. J. Membrane Biol. 190: 1–14.
Kocal, T., Quinn, B.A., Smith, I.R., Ferguson, H.W. and Hayes, M.A. 1988. Use of trout serum to prepare primary attached monolayer cultures of hepatocytes from rainbow trout (Salmo gairdneri). In Vitro Cell. Dev. Biol. 24: 304–308.
Kültz, D. and Somero, G.N. 1996. Differences in protein patterns of gill epithelial cells of the fish Gillichthys mirabilis after osmotic and thermal acclimation. J. Comp. Physiol B: 88–100.
Lannan, C.N., Winton, J.R. and Fryer, J.L. 1984. Fish cell lines: establishment and characterization of nine cell lines from salmonids. In Vitro Cell Dev. Biol. 20: 671–676.
Liebler, D.C. 2002. Introduction to Proteomics: Tools for a New Biology. Humana Press, Totowa, New Jersey. 198 pp.
Maitre, J-L., Valotaire, Y. and Guguen-Guillouzo, C. 1986. Estradiol-17 β stimulation of vitellogenin synthesis in primary culture of male rainbow trout hepatocytes. In Vitro Cell. Dev. Biol. 22: 360–362.
Marion, M. and Denizeau, F. 1983. Rainbow trout and human cells in culture for the evaluation of the toxicity of aquatic pollutants: a study with cadmium. Aquat. Toxicol. 3: 329–343.
Marmorstein, A.D., Mortell, K.H., Ratcliffe, D.R. and Cramer, E.B. 1992. Epithelial permeability factor: a serum protein that condenses actin and opens tight junctions. Am. J. Physiol. 262: C1403–C1410.
Mitani, H., Naruse, K. and Shima, A. 1989. Eurythermic and stenothermic growth of cultured fish cells and their thermosensitivity. J. Cell. Sci. 93: 731–737.
Pärt, P. and Bergström, E. 1995. Primary cell cultures of teleost branchial epithelial cells. In: Cellular and Molecular Approaches to Fish Ionic Regulation, pp. 207–227. Edited by Wood, C.M. and Shuttleworth, T.J. Academic Press, San Diego.
Pärt, P., Norrgren, L., Bergström, E. and Sjoberg, E. 1993. Primary cultures of epithelial cells from rainbow trout gills. J. Exp. Biol. 175: 219–232.
Pandey, A. and Mann, M. 2000. Proteomics to study genes and genomes. Nature 405: 837–846.
Plumb, J.A. and Wolf, K. 1971. Fish cell growth rates. Quantitative comparison of RTG-2 Cell growth at 5–25 °C. In Vitro Cell Dev. Biol. 7: 42–45.
Rachlin, J.W. and Perlmutter, A. 1968. Fish cells in culture for study of aquatic toxicants. Water Res. 2: 409–414.
Sandbacka, M., Pärt, P. and Isomaa, B. 1999. Gill epithelial cells as tools for toxicity screening - comparison between primary cultures, cells in suspension and epithelia on filters. Aquat. Toxicol. 46: 23–32.
Smith, R.W., Jönsson, M., Houlihan, D.F. and Pärt, P. 2001. Minimising aerobic respiratory demands could form the basis to sub-lethal copper tolerance by rainbow trout gill epithelial cells in vitro. Fish Physiol. Biochem. 24: 157–169.
Soivio, A., Westman, K. and Nyholm, K. 1972. Improved method of dorsal aorta catherization: Haematological effects followed for three weeks in rainbow trout (Salmo gairdneri). Finn. Fish. Res. 1: 11–21.
Sprague, J.B. 1969. Measurement of pollutant toxicity to fish - I. Bioassay methods for acute toxicity. Water Res. 3: 793–821.
Tsugawa, K. and Lagerspetz, K.Y.H. 1990. Direct adaptation of cells to temperature: membrane fluidity of goldfish cells cultured in vitro at different temperatures. Comp. Biochem. Physiol. 96A: 57–60.
Tsugawa, K. and Takahashi, K.P. 1987. Direct adaptation of cells to temperature: cold-stable microtubule in rainbow trout cells cultured in vitro at low temperature. Comp. Biochem. Physiol. 87A: 745–748.
Walsh, S.V., Hopkins, A.M. and Nusrat, A. 2000. Modulation of tight junction structure and function by cytokines. Advanced Drug Delivery Reviews 41: 303–313.
Wolf, K. and Quimby, M.C. 1962. Established eurythermic line of fish cells in vitro. Science 135: 1065–1066.
Wolf, K. and Quimby, M.C. 1969. Fish cell and tissue culture. In: Fish Physiology, Vol. 3. pp. 253–305. Edited by Hoar, W.S. and Randall, D.J. Academic Press, New York.
Wood, C.M. and Pärt, P. 1997. Cultured branchial epithelia from freshwater fish gills. J. Exp. Biol. 200: 1047–1059.
Wood, C.M., Gilmour, K.M. and Pärt, P. 1998. Passive and active transport properties of a gill model, the cultured branchial epithelium of the freshwater rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. 119A: 87–96.
Wood, C.M., Kelly, S.P., Zhou, B., Fletcher, M., O'Donnell, M., Eletti, B. and Pärt, P. 2002. Cultured gill epithelia as models for the freshwater fish gill. Biochim. Biophys. Acta (BBA) - Biomembranes 1566: 72–83.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wood, C., Eletti, B. & Pärt, P. New methods for the primary culture of gill epithelia from freshwater rainbow trout. Fish Physiology and Biochemistry 26, 329–344 (2002). https://doi.org/10.1023/B:FISH.0000009262.45438.79
Issue Date:
DOI: https://doi.org/10.1023/B:FISH.0000009262.45438.79