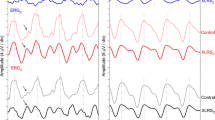
Abstract
Dark and light adapted ERGs were recorded in 19 patients with XLRS and in 15 patients with CSNB. Patients were assigned to clinical groups after identification of mutations in the RS1 (16 patients), NYX (11 patients) and CACNA1F (4 patients) genes causing XLRS, 'complete' CSNB and 'incomplete' CSNB, respectively. ERG responses were compared with those of 26 healthy volunteers. Rod responses were most severely affected in patients in the NYX group but a rod-generated b-wave could be identified in the majority of patients in this group. Rod responses were less severely affected in the CACNA1F and RS1 groups and ERGs did not differ significantly between these two groups. Cone reponses were largely unaffected in the NYX group but were abnormal in the RS1 and especially CACNA1F groups. The ERG results suggest that the RS1 and CACNA1F gene products have comparable functional consequences and that all three genes may affect multiple retinal sites.
Similar content being viewed by others
References
Peachey NS, Fishman GA, Derlacki D, Brigell MG. Psychophysical and electroretinographic findings in X-linked juvenile retinoschisis. Arch Ophthalmol 1987; 105: 513–16.
Kellner U, Brummer S, Foerster MH, Wessing A. X-linked congenital retinoschisis. Graefe's Arch Clin Exp Ophthalmol 1990; 228: 432–37.
Bradshaw K, George N, Moore AT, TrumpD. Mutations of the XLRS1 gene cause abnormalities of photoreceptor as well as inner retinal responses of the ERG. Doc Ophthalmol 1999; 98: 153–73.
Miyake Y, Yagasaki K, Horiguchi M, Kawase Y, Kanda T. Congenital stationary night blindness with negative electroretinogram. Arch Ophthalmol 1986; 104: 1013–20.
Miyake Y, Horiguchi M, Ora I, Shiroyama N. Characteristic flicker anomaly in incomplete stationary night blindness. Invest Ophthalmol Vis Sci 1987; 28: 1816–23.
Tremblay F, Laroch RG, Becker ID. The electroretinographic disgnosis of the incomplete form of congenital stationary night blindness. Vision Res 1995; 16: 2383–93.
Allen LE, Zito I, Bradshaw K, Paterl RJ, Bird AC, Fitzke F, Yates JR, TrumpD, Hardcastle A, Moore AT. Genotype-phenotype correlation in British families with X-linked congenital stationary night blindness. Br J Ophthalmol 2003; in press.
The Retinoschisis Consortium:. Functional implications of the spectrum of mutations found in 234 cases with X-linked juvenile retinoschisis (XLRS 1). Hum Mol Genet 1998; 7: 1185–92.
Zito I, Allen LE, Patel RJ, Meindl A, Bradshaw K, Yates JR, Bird AC, Erskin L, Cheetham ME, Webster AR, Poopalasundaram S, Moore AT, Trump D, Hardcastle A. Mutations in the CACNA1F and NYX genes in British CSNBX families. Hum Mutat 2003; 21: 169.
Marmor MF, Zrenner E. Standard for clinical electroretinography (1999 update). Doc Opthalmol 1998/1999; 97: 143–56.
TrumpD, Grayson C, Sowden J, Ellis J, Bobrow LG, Yates JRW. X-linked retinoschisis: Investigations of the XLRS1 expression pattern and characterisation of a novel antibody against the XLRS1 protein. Invest Ophthalmol Vis Sci 1999; 40: S961.
Pusch CM, Zeitz C, Brandau O, Pesch K, Achatz H, Feil S, Scharfe C, Maurer J, Jacobi FK, Pinckers A, Andreasson S, Hardcastle A, Wissinger B, Berger W, Meindle A. The complete form of X-linked congenital stationary night blindness is caused by mutations in a gene encoding a leucine-rich repeat protein. Nature Gen 2000; 26: 324–27.
Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, KoopB, Fishman G, Mets M, Musarrella MA, Boycott KM. Mutations in NYX encoding the leucine-rich proteoglycan nyctalopin cause X-linked complete congenital stationary night blindness. Nat Genet 2000; 26: 319–23.
Strom TM, Nyakatura C, Apfelsted-Sylla E, Hellebrand H, Lorentz B, Weber BH et al. An L-type calcium-channel gene mutuated in incomplete X-linked congenital stationary night blindness. Nat Genet 1998; 19: 260–63.
Nakamura M, Ito S, Terasaki H, Miyake Y. Novel CACNA1F mutations in Japanese patients with incomplete stationary night blindness. Invest Ophthalmol Vis Sc 2001; 42: 1610–16.
Bradshaw K, Newman D, Allen L, Moora A. Abnormalities of the scotopic thereshold response correlated with gene mutation in X-linked retinoschisis ans congenital stationary night blindness. Doc Ophthalmol 2003; in press.
Sieving PA, Bingham EL, KempJ, Richards J, Hiriyanna K. Juvenile X-linked retinoschisis from XLRS1 Arg213Trp mutation with preservation of the electroretinogram scotopic b-wave. Am J Opthalmol 1999; 128: 179–84.
Robson JG, Frishman LJ. Dissecting the dark-adapted electroretinogram. Doc Ophthalmol 1998/1999; 95:187-15.
Scholl HPN, Langrova H, Pusch CM, Wissinger B, Zrenner E, Apfelstedt-Sylla E. Slow and fast rod ERG pathways in patients with X-linked complete stationary night blindness carrying mutations in the NYX gene. Invest Ophthalmol Vis Sc 2001; 42: 2728–36.
Molday LL, Hicks D, Sauer CG, Sauer CG, Champliaud-Steiner MF, Weber BHF, Molday RS. Expression of X-linked retinoschisin protein in photoreceptor and bipolar cells. Invest Ophthalmol Vis Sci 2001; 42: 816–25.
Lachapelle P, Little JM, Polomeno RC. The photopic electroretinogram in congenital stationary night blindness with myopia. Invest Opthalmol Vis Sci 1983; 24: 442–50.
Kim SH, Bush RA, Sieving PA. Increased phase lag of the fundamental harmonic component of the 30Hz flicker ERG in Schubert-Bornschein complete type CSNB. Vision Res 1997; 37: 2471–75.
Alexander KR, Fishman GA, Barnes CS, Grover S. Onresponse deficit in the electroretinogram of the cone system in X-linked retinoschisis. Invest Ophthalmol Vis Sci 2001; 42: 453–59.
Khan NW, Jaminson JA, KempJA, Sieving PA. Analysis of photoreceptor function and inner retinal activity in juvenile X-linked retinoschisis. Vision Res 2001; 41: 3931–42.
Miyake Y, Yagasaki K, Horiguchi M, Kawase Y. ON-and OFF-responses in photopic electroretinogram in complete and incomplete types of congenital stationary night blindness. Jpn J Opthalmol 1987; 31: 81–87.
Bush RA, Sieving PA. A proximal retinal contribution to the primate photopic a-wave. Invest Ophthalmol Vis Sci 1994; 35: 635–45.
Mason PJ, Allen CP, Friedburg C, Lamb TD. Photoreceptor and post-receptoral signals in the photopic a-wave of the human ERG. J Physiol 2001; 531P: 85P.
Robson JG, Saszik SM, Ahmed J, Frishman LJ. Rod and cone contributions to the a-wave of the electroretinogram of the macaque. J Physiol 2003; 547.2: 509–30.
Kondo M, Sieving PA. Primate photopic sine-wave flicker ERG: vector modeling analysis of component origins using glutamate analogs. Invest Opthalmol Vis Sci 2001; 42: 305–12.
Viswanathan S, Frishman LJ, Robson JG, Harwerth RS, Smith EL. The Photopic Negative Response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci 1999; 40: 1124–36.
Sieving P. Photopic on-and off-pathway abnormalities in retinal dystrophies. Trans Am Opthalmol Soc 1993; 81: 701–73.
Quigley M, Roy MS, Barsoum-Homsey L, Chevrette JL, Milot J. ON-and off-responses in the photopic electroretinogram in complete-type congenital stationary night blindness. Doc Opthalmol 1996/1997; 92: 159–65.
Langrova H, Gamer D, Friedburg C, Besch D, Zrenner E, Apfelstedt-Sylla E. Abnormalities of the long flash ERG in congenital stationary night blindness of the Schubert-Bornschein type. Vision Res 2002; 42: 1475–83.
Alexander KR, Barnes CS, Fishman GA. Origin of deficits in the flicker electroretinogram of the cone system in X-linked restinoschisis as derived from repsonse non-linearities. J Opt Soc Am 2001; 18: 747–54.
Alexander KR, Barnes CS, Fishman GA. High frequency attenuation of the cone ERG and on-response deficit in X-linked retinoschisis. Invest Ophthalmol Vis Sc 2001; 42:2094-01.