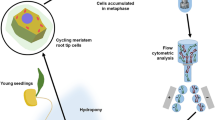
Abstract
The application of flow cytometry and sorting (flow cytogenetics) to plant chromosomes did not begin until the mid-1980s, having been delayed by difficulties in preparation of suspensions of intact chromosomes and discrimination of individual chromosome types. These problems have been overcome during the last ten years. So far, chromosome analysis and sorting has been reported in 17 species, including major legume and cereal crops. While chromosome classification by flow cytometry (flow karyotyping) may be used for quantitative detection of structural and numerical chromosome changes, chromosomes purified by flowsorting were found to be invaluable in a broad range of applications. These included physical mapping using PCR, high-resolution cytogenetic mapping using FISH and PRINS, production of recombinant DNA libraries, targeted isolation of markers, and protein analysis. A great potential is foreseen for theuse of sorted chromosomes for construction of chromosome and chromosome-arm-specific BAC libraries, targeted isolation of low-copy (genic) sequences, high-throughput physical mapping of ESTs and other DNA sequences by hybridization to DNA arrays, and global characterization of chromosomal proteins using approaches of proteomics. This paper provides a comprehensive review of the methodology and application of flow cytogenetics, and assesses its potential for plant genome analysis.
Similar content being viewed by others
References
Arumuganathan K, Slattery JP, Tanksley SD, Earle ED (1991) Preparation and flow cytometric analysis of metaphase chromosomes of tomato. Theor Appl Genet 82: 101–111.
Arumuganathan K, Martin GB, Telenius H, Tanksley SD, Earle ED (1994) Chromosome 2-specific DNA clones from flow-sorted chromosomes of tomato. Mol Gen Genet 242: 551–558.
Binarová P, Hause B, Doležel J, Dráber P (1998) Association of g-tubulin with kinetochore/centromeric region of plant chromosomes. Plant J 14: 751–757.
Boschman GA, Manders EMM, Rens W, Slater R, Aten JA (1992) Semi-automated detection of aberrant chromosomes in bivariate flow karyotypes. Cytometry 13: 469–477.
Carrano AV, Gray JW, Langlois RG, Yu LC (1983) Flow cytogenetics: Methodology and applications. In: Rowley JD, Ultmann JE, eds. Chromosomes and Cancer. New York: Academic Press, Inc., pp 195–209.
Cenci A, Chantret N, Kong X et al. (2003) Construction and characterization of a half million clone BAC library of durum wheat (Triticum turgidum ssp. durum). Theor Appl Genet 107: 931–939.
Conia J, Bergounioux C, Perennes C, Muller P, Brown S, Gadal P (1987) Flow cytometric analysis and sorting of plant chromosomes from Petunia hybrida protoplasts. Cytometry 8: 500–508.
Conia J, Muller P, Brown S, Bergounioux C, Gadal P (1989) Monoparametric models of flow cytometric karyotypes with spreadsheet software. Theor Appl Genet 77: 295–303.
Cremer T, Lichter P, Borden J, Ward DC, Manuelidis L (1988) Detection of chromosome aberrations in metaphase and interphase tumor cells by in situ hybridisation using chromosome specific library probes. Hum Genet 80: 235–246.
de Jong G, Telenius AH, Telenius H, Perez CF, Drayer JI, Hadlaczky G (1999) Mammalian artificial chromosome pilot facility: Large-scale isolation of functional satellite DNA-based artificial chromosomes. Cytometry 35: 129–133.
de Jong JH, Fransz P, Zabel P (1999) High resolution FISH in plants ‐ techniques and applications. Trends Plant Sci 4: 258–263.
de Laat AMM, Blaas J (1984) Flow-cytometric characterization and sorting of plant chromosomes. Theor Appl Genet 67: 463–467.
de Laat AMM, Schel JHN(1986) The integrity of metaphase chromosomes of Haplopappus gracilis (Nutt.) Gray isolated by flow cytometry. Plant Sci 47: 145–151.
Doležel J (1991) KARYOSTAR: Microcomputer program for modelling of monoparametric flow karyotypes. Biológia 46: 1059–1064.
Doležel J, Lucretti S (1995) High-resolution flow karyotyping and chromosome sorting in Vicia faba lines with standard and reconstructed karyotypes. Theor Appl Genet 90: 797–802.
Doležel J, Binarová P, Lucretti S (1989) Analysis of nuclear DNA content in plant cells by flow cytometry. Biol Plant 31: 113–120.
Doležel J, Číhalíková J, Lucretti S (1992) A high-yield procedure for isolation of metaphase chromosomes from root tips of Vicia faba L. Planta 188: 93–98.
Doležel J, Lucretti S, Schubert I (1994) Plant chromosome analysis and sorting by flow cytometry. Crit Rev Plant Sci 21: 275–309.
Doležel J, Číhalíková J, Weiserová J, Lucretti S (1999a) Cell cycle synchronization in plant root meristems. Meth Cell Sci 21: 95–107.
Doležel J, Macas J, Lucretti S (1999b) Flow analysis and sorting of plant chromosomes. In: Robinson JP, Darzynkiewicz Z, Dean PN et al., eds. Current Protocols in Cytometry. New York: John Wiley and Sons, Inc., pp 5.3.1.–5.3.33.
Doležel J, Lysák MA, Kubaláková M, Šimková H, Macas J, Lucretti S (2001) Sorting of plant chromosomes. In: Darzynkiewicz Z, Crissman HA, Robinson JP, eds. Flow Cytometry, 3rd Edition, Part B. San Diego: Academic Press, pp 3–31.
Eriksson T (1966) Partial synchronization of cell division in suspension cultures of Haplopappus gracilis. Physiol Plant 19: 900–910.
Gill KS, Arumuganathan K, Le JH (1999) Isolating individual wheat (Triticum aestivum) chromosome arm by flow cytometric analysis of ditelosomic lines. Theor Appl Genet 98: 1248–1252.
Givan AL (2001) Principles of flow cytometry: an overview. In: Darzynkiewicz Z, Crissman HA, Robinson JP, eds. Flow Cytometry, 3rd Edition, Part A. San Diego: Academic Press, pp 19–50.
Gray JW, Cram LS (1990) Flow karyotyping and chromosome sorting. In: Melamed MR, Lindmo T, Mendelsohn ML, eds. Flow Cytometry and Sorting, Second Edition. New York: Wiley-Liss, pp 503–529.
Grunwald D, Frelat G, Vaiman M (1989) Animal flow cytogenetics. In: Yen A, ed. Flow Cytometry: Advanced Research and Clinical Applications, Vol. 1. Boca Raton: CRC Press, Inc., pp 132–140.
Gualberti G, Doležel J, Macas J, Lucretti S (1996) Preparation of pea (Pisum sativum L.) chromosome and nucleus suspensions from single root tips. Theor Appl Genet 92: 744–751.
Kejnovsk_y E, Vrána J, Matsunaga S et al. (2001) Localization of male-specifically expressed MROS genes of Silene latifolia by PCR on flow-sorted sex chromosomes and autosomes. Genetics 158: 1269–1277.
Koblížková A, Doležel J, Macas J (1998) Subtraction with 3' modified oligonucleotides eliminates amplification artifacts in DNA libraries enriched for microsatellites. BioTechniques 25: 32–38.
Kubaláková M, Lysák MA, Vrána J, Šimková H, Číhalíková J, Doležel J (2000) Rapid identification and determination of purity of flow-sorted plant chromosomes using C-PRINS. Cytometry 41: 102–108.
Kubaláková M, Vrána J, Číhalíková J, Šimková H, Doležel J (2002) Flow karyotyping and chromosome sorting in bread wheat (Triticum aestivum L.). Theor Appl Genet 104: 1362–1372.
Kubaláková M, Valárik M, Bartoš J et al. (2003a) Analysis and sorting of rye (Secale cereale L.) chromosomes using flow cytometry. Genome 46: 893–905.
Kubaláková M, Bartoš J, Ková›ová P et al. (2003b) Chromosome analysis and sorting in durum wheat and physical mapping of repetitive DNA sequences. In: Proceedings of the Tenth International Wheat Genetics Symposium. Istituto Sperimentale per la Cerealicoltura, Paestum (in press).
Lee JH, Arumuganathan K (1999) Metaphase chromosome accumulation and flow karyotypes in rice (Oryza sativa L.) root tip meristem cells. Mol Cells 9: 436–439.
Lee JH, Arumuganathan K, Kaeppler SM, Kaeppler HF, Papa CM (1996) Cell synchronization and isolation of metaphase chromosomes from maize (Zea mays L.) root tips for flow cytometric analysis and sorting. Genome 39: 697–703.
Lee JH, Arumuganathan K, Yen Y, Kaeppler S, Kaeppler H, Baenziger PS (1997) Root tip cell cycle synchronization and metaphase-chromosome isolation suitable for flow sorting in common wheat (Triticum aestivum L.). Genome 40: 633–638.
Lee JH, Arumuganathan K, Chung YS et al. (2000) Flow cytometric analysis and chromosome sorting of barley (Hordeum vulgare L.). Mol Cells 10: 619–625.
Lee JH, Arumuganathan K, Kaeppler SM et al. (2002) Variability of chromosomal DNA contents in maize (Zea mays L.) inbred and hybrid lines. Planta 215: 666–671.
Leitch AR, Schwarzacher T, Wang ML et al. (1993) Molecular cytogenetic analysis of repeated sequences in a long-term wheat-suspension culture. Plant Cell Tissue Org Cult 33: 287–296.
Li L, Arumuganathan K (2000) High recovery of large molecular weight DNA from sorted maize chromosomes. Plant Mol Biol Rep 18: 41–45.
Li LJ, Arumuganathan K, Rines HW et al. (2001) Flow cytometric sorting of maize chromosome 9 from an oatmaize chromosome addition line. Theor Appl Genet 102: 658–663.
Lucretti S, Doležel J (1997) Bivariate flow karyotyping in broad bean (Vicia faba). Cytometry 28: 236–242.
Lucretti S, Doležel J, Schubert I, Fuchs J (1993) Flow karyotyping and sorting of Vicia faba chromosomes. Theor Appl Genet 85: 665–672.
Lysák MA, Číhalíková J, Kubaláková M, Šimková H, Künzel G, Doležel J (1999) Flow karyotyping and sorting of mitotic chromosomes of barley (Hordeum vulgare L.). Chromosome Res 7: 431–444.
Macas J, Doležel J, Lucretti S et al. (1993) Localization of seed protein genes on flow-sorted field bean chromosomes. Chromosome Res 1: 107–115.
Macas J, Doležel J, Gualberti G, Pich U, Schubert I, Lucretti S (1995) Primer-induced labeling of pea and field bean chromosomes in situ and in suspension. BioTechniques 19: 402–408.
Macas J, Gualberti G, Nouzova´ M, Samec P, Lucretti S, Doležel J (1996) Construction of chromosome-specific DNA libraries covering the whole genome of field bean (Vicia faba L.). Chromosome Res 4: 531–539.
Mayer K, Mewes HW (2002) How can we deliver the large plant genomes? Strategies and perspectives. Curr Opin Plant Biol 5: 173–177.
Neumann P, Lysák M, Doležel J, Macas J (1998) Isolation of chromosomes from Pisum sativum L. hairy root cultures and their analysis by flow cytometry. Plant Sci 137: 205–215.
Neumann P, Požárková D, Vrána J, Doležel J, Macas J (2002) Chromosome sorting and PCR-based physical mapping in pea (Pisum sativum L.). Chromosome Res 10: 63–71.
Pich U, Meister A, Macas J, Doležel J, Lucretti S, Schubert I (1995) Primed in situ labelling facilitates flow sorting of similar sized chromosomes. Plant J 7: 1039–1044.
Pinkel D, Landegent J, Collins C et al. (1988) Fluorescence in situ hybridization with human chromosome-specific libraries. Detection of trisomy 21 and translocations of chromosome 4. Proc Natl Acad Sci USA 85: 9138–9142.
Požárková D, Koblížková A, Román B et al. (2002) Development and characterization of microsatellite markers from chromosome 1-specific DNA libraries of Vicia faba. Biol Plant 45: 337–345.
S›afá› J, Janda J, Bartoš J et al. (2003) Dissecting the complex genome of wheat: construction of a chromosome-specific BAC library. Genome Res (submitted).
Schubert I, Doležel J, Houben A, Scherthan H, Wanner G (1993) Refined examination of plant metaphase chromosome structure at different levels made feasible by new isolation methods. Chromosoma 102: 96–101.
Schubert I, Fransz PF, Fuchs J, de Jong H (2003) Chromosome painting in plants. Meth Cell Sci 23: 57–69.
Schwarzacher T, Wang ML, Leitch AR, Miller N, Moore G, Heslop-Harrison JS (1997) Flow cytometric analysis of the chromosomes and stability of a wheat cell-culture line. Theor Appl Genet 94: 91–97.
Šimková H, Číhalíková J, Vrána J, Lysák MA, Doležel J (2003) Preparation of high molecular weight DNA from plant nuclei and chromosomes isolated from root tips. Biol Plantarum 46: 369–373
Telenius H, Carter NP, Bebb, CE., Nordenskjöld M, Ponder BAJ, Tunnacliffe A (1992). Degenerate oligonucleotideprimed PCR: general amplification of target DNA by a single degenerate primer. Genomics 13: 718–725.
ten Hoopen R, Manteuffel R, Doležel J, Malysheva L, Schubert I (2000) Evolutionary conservation of kinetochore protein sequences in plants. Chromosoma 109: 482–489.
Ñberall I, Vrána J, Bartoš J, Šmerda J, Doležel J, Havel L (2003) Isolation of chromosomes from Picea abies L. and their analysis by flow cytometry. Biol Plant (in press).
Valárik M, Bartoš J, Ková›ová P, Kubaláková M, de Jong JH, Doležel J (2003) High-resolution FISH of super-stretched flow sorted plant chromosomes. Plant J (submitted).
Van Dilla MA, Deaven LL, Albright KL et al. (1986) Human chromosome-specific DNA libraries. Construction and availability. Biotechnology 4: 537–552.
Vaz Patto MC, Torres AM, Koblížková A, Macas J, Cubero JI (1999) Development of a genetic composite map of Vicia faba using F2 populations derived from trisomic plants. Theor Appl Genet 98: 736–743
Veuskens J, Marie D, Brown SC, Jacobs M, Negrutiu I (1995) Flow sorting of the Y sex-chromosome in the dioecious plant Melandrium album. Cytometry 21: 363–373.
Vlá›ilová K, Ohri D, Vrána J et al. (2002) Development of flow cytogenetics and physical genome mapping in chickpea (Cicer arietinum L.). Chromosome Res 10: 695–706.
Vrána J, Kubaláková M, Šimková H, Číhalíková J, Lysák MA, Doležel J (2000) Flow-sorting of mitotic chromosomes in common wheat (Triticum aestivum L). Genetics 156: 2033–2041.
Wang ML, Leitch AR, Schwarzacher T, Heslop-Harrison JS, Moore G (1992) Construction of a chromosome-enriched HpaII library from flow-sorted wheat chromosomes. Nulecic Acids Res 20: 1897–1901.
Wanner G, Formanek H, Martin R, Herrmann RG (1991) High resolution scanning electron microscopy of plant chromosomes. Chromosoma 100: 103–109.
Waugh R, Dear PH, Powell W, Machray GC (2002) Physical education-new technologies for mapping plant genomes. Trends Plant Sci 7: 521–523.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Doležel, J., Kubaláková, M., Bartoš, J. et al. Flow cytogenetics and plant genome mapping. Chromosome Res 12, 77–91 (2004). https://doi.org/10.1023/B:CHRO.0000009293.15189.e5
Issue Date:
DOI: https://doi.org/10.1023/B:CHRO.0000009293.15189.e5