Abstract
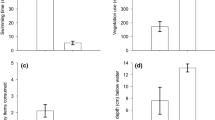
Dispersal is a key element of a species' invasiveness. Although considerable work has addressed how dispersal influences the pattern of spatial spread of invading organisms, few studies investigate whether invasive species are in fact better dispersers than either the species they displace or less successful invaders. Recent work suggests that variation in dispersal may be due to variation in an underlying behavioral trait, boldness. Our study examined the link between dispersal, boldness, and invasiveness by comparing the dispersal characteristics and refuge use of two invasive Gambusia species to two congeners in experimental streams. The streams consisted of a series of pools (no flow) connected to a flowing channel. For each species, small groups of females were released at the middle pool, and their movement and activity were recorded over a 1-h period. We found invasive Gambusia to be more likely to disperse out of the introductory pool, to disperse sooner, to travel a greater distance in the artificial streams, and thus to exhibit greater dispersal tendencies than their close relatives. Among the invasives, Gambusia affinis had a greater dispersal tendency than G. holbrooki. We suspect this result indicates variation in the contribution of dispersal to the relative invasiveness of these species. Certain dispersal measures were correlated to time spent out of refuge, although invasive Gambusia and their relatives did not differ in the predicted manner. These results argue for the greater incorporation of experimental approaches and analyses of behavioral mechanisms in the study of invasive species.
Similar content being viewed by others
References
Andow DA, Kareiva PM, Levin SA and Okubo A (1990) Spread of invading organisms.Landscape Ecology 4: 177–188
Arthington AH and Lloyd LN (1989)Introduced poeciliids in Australia and New Zealand. In:Meffe GK and Snelson FF (eds) Ecology and Evolution of Livebearing Fishes (Poeciliidae),pp 333–348. Prentice Hall, Englewood Cliffs, New Jersey
Bengtsson G, Hedlund K and Rundgren S (1994) Food-dependent and density-dependent dispersal: evidence from a soil collembolan. Journal of Animal Ecology 63: 513–520
Bradford MJ and Taylor GC (1997) Individual variation indispersal behaviour of newly emerged chinook salmon (On-corhynchus tshawytscha) from the Upper Fraser River, British Columbia. Canadian Journal of Fisheries and Aquatic Sciences 54: 1585–1592
Brown KL (1985) Demographic and genetic characteristics of dispersal in the mosquito sh, Gambusia a.nis (Pisces:Poeciliidae).Copeia 1985: 597–612
Brown KL (1987) Colonization by mosquito sh (Gambusia a.-inis) of a Great Plains River Basin. Copeia 1987: 336–351
Burgess GH and Franz R (1989) Zoogeography of the Antil-lean freshwater sh fauna.In: Woods CA(ed) Biogeography of the West Indies, 263–304. Sandhill Crane Press, Gainesville, Florida
Casterlin ME and Reynolds WW (1977) Aspects of habitat selection in the mosquito sh Gambusia a.nis.Hydrobiologia 55:125–127
Chesser RK, Smith MW and Smith MH (1984) Biochemical genetics of mosquito sh. III. Incidence and significance of multiple insemination.Genetica 64:77–81
Coleman K and Wilson DS (1998) Shyness and boldness in pumpkinseed sun sh:individual differences are context-specific.Animal Behaviour 56:927–936
Congdon BC (1994) Characteristics of dispersal in the eastern mosquito sh Gambusia holbrooki. Journal of Fish Biology 45:943–952
Congdon BC (1995) Unidirectional gene.ow and maintenance of genetic diversity in mosquito sh Gambusia holbrooki (Teleostei:Poeciliidae). Copeia 1995:162–172
Courtenay WR and Meffe GK (1989) Small fishes in strange places: a review of introduced Poeciliids.In: Meffe GK and Snelson FF (eds) Ecology and Evolution of Live bearing Fishes (Poeciliidae),pp 319–331.Prentice Hall, Englewood Cliffs, New Jersey
Duncan RP, Bomford M, Forsyth DM and Conibear L (2001) High predictability in introduction outcomes and the geographic range size of introduced Australian birds: a role of climate.Journal of Animal Ecology 70:621–632
Ehrlich PR (1986) Which animal will invade? In: Mooney HA and Drake JA (eds) Ecology of Biological Invasions of North American and Hawaii, pp 79–95. Springer-Verlag, New York
Endler JA (1977) Geographic Variation,Speciaton and Clines. Princeton University Press, Princeton, New Jersey, 262 pp
Fraser DF, Gilliam JF, Daley MJ, Le AN and Skalski GT (2001) Explaining leptokurtic movement distributions: intrapopulation variation in boldness and exploration. American Naturalist 158:124–135
Fuller PL, Nico LG and Williams JD (1999) Nonindigenous Fishes Introduced into Inland Waters of the United States. American Fisheries Society, Special Publication 27, Bethesda, Maryland, 613 pp
Gaines MS and McClenaghan Jr LR (1980) Dispersal in small mammals. Annual Review of Ecology and Systematics 11: 163–196
Gammon DE and Maurer BA (2002) Evidence for non-uniform dispersal in the biological invasions of two naturalized North American bird species. Global Ecology and Biogeography 11:1155–1161
Gamradt SC and Kats LB (1996) Effect of introduced cray sh and mosquito sh on California newts. Conservation Biology 10:1155–1162
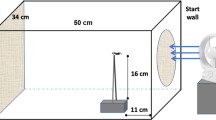
Gelwick FP and Matthews WJ (1993) Artificial streams for studies of fish ecology. Journal of the North American Benthological Society 12:343–347
Goodsell JA and Kats LB (1999) Effect of introduced mosquito fish on pacific tree frogs and the role of alternative prey. Conservation Biology 13:921–924
Greenberg R (1989) Neophobia;aversion to open space, and ecological plasticity in song and swamp sparrows. Canadian Journal of Zoology 67: 1194–1199
Greenberg R (1995) Novelty responses: the bridge between psychology, behavioral ecology and community ecology. Trends in Ecology and Evolution 10:165–166
Greenwood PJ and Harvey PH (1976) The adaptive significance of variation in breeding area delity of the blackbird (Turdus merula L.). Journal of Animal Ecology 45:887–898
Hanski WE and Gilpin M (1991) Metapopulation dynamics: brief history and conceptual domain. Biological Journal of the Linnean Society 42:3–16
Higgins SI and Richardson DM (1999) Predicting plant migration rates in a changing world: the role of long-distance dispersal. American Naturalist 153:464–475
Holway DA and Suarez AV (1999) Animal behavior: an essential component of invasion biology. Trends in Ecology and Evolution 14:328–330
Howe HF and Westley LC (1986) Ecology of pollination and seed dispersal.In:Crawley MJ (ed) Plant Ecology, 185–216. Blackwell Scientific, Oxford,UK
Hubbs CL and Lagler KF (1964) Fishes of the Great Lakes Region. University of Michigan Press, Ann Arbor, Michigan, 213 pp
Hubbs C and Springer VG (1957) A revision of the Gambusia nobilis species group, with descriptions of three new species, and notes on their variation, ecology, and evolution. Texas Journal of Science 9:279–327
Hynes HBN (1970) The Ecology of Running Waters. Liver-pool University Press, Liverpool, UK, 555 pp
ISSG (2000) 100 of the World' s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database. Invasive Species Specialist Group (IUCN).University of Auckland, Auckland, New Zealand, 11 pp
Johnson LE and Carlton JT (1996) Post-establishment spread in large-scale invasions: dispersal mechanisms of the zebra mussel Dreissena polymorpha. Ecology 77:1686–1690
Kareiva PM (1990) Population dynamics in spatially complex environments: theory and data. Philosophical Transactions of the Royal Society of London B 330:175–190
Kolar CS and Lodge DM (2001) Progress in invasion biology: predicting invaders.Trends in Ecology and Evolution 16:199–204
Kolar CS and Lodge DM (2002) Ecological predictions and risk assessment for alien fishes in North America.Science 298:1233–1236
Kot M, Lewis MA and van den Driessche P (1996) Dispersal data and the spread of invading organisms.Ecology 77:2027–2042
Lever C (1996) Naturalized Fishes of the World. Academic Press, New York, 408 pp
Lloyd LN, Arthington AH and Milton DA (1986) The mosquito sh-a valuable mosquito-control agent or a pest? In: Kitching RL (ed) The Ecology of Exotic Animals and Plants: Some Australian Case Histories, 6–27. J. Wiley & Sons, Brisbane, Australia
Lodge DM (1993a) Biological invasions: lessons for ecology. Trends in Ecology and Evolution 8:133–137
Lodge DM (1993b) Species invasions and deletions:community effects and responses to climate and habitat change. In: Kareiva PM, Kingsolver JG and Huey RB (eds) Biotic Interactions and Global Change, 367–387.Sinauer
Sunderland, Massachusetts Lydeard C, Wooten MC and Meyer A (1995) Molecules, morphology,and area cladograms:a cladistic and biogeographic analysis of Gambusia (Teleostei:Poeciliidae). Systematic Biology 44:221–236
Mack RN (1996) Predicting the identity and fate of plant invaders: emergent and emerging approaches. Biological Conservation 78:107–121
Mack RN, Simberlo.D, Lonsdale WM, Evans H, Clout M and Bazzaz FA (2000) Biotic invasions:causes, epidemiology, global consequences, and control. Ecological Applications 10:689–710
Marchetti MP, Moyle PB and Levine R (2004) Alien fishes in California watersheds:characteristics of successful and failed invaders.Ecological Applications 14:587–596
Moyle PB (1986) Fish introductions into North America: patterns and ecological impact.In: Mooney HA and Drake JA (eds) Ecology of Biological Invasions of North America and Hawaii, 27–43. Springer-Verlag, New York
Okubo A (1980) Diffusion and Ecological Problems: Mathematical Models. Springer-Verlag, Berlin, Germany,254 pp
O' Riain MJ, Jarvis JU and Faulkes CG (1996) A dispersive morph of the naked mole-rat. Nature 380:619–621
Paradis E, Baillie SR, Sutherland WJ and Gregory RD (1998) Patterns of natal and breeding dispersal in birds. Journal of Animal Ecology 67:518–536
Parker IM and Reichard SH (1998)Critical issues in invasion biology for conservation science. In: Fiedler PL and Kareiva PM (eds)Conservation Biology for the Coming Decade, 2nd edn, pp 283–305.Chapman and Hall, New York
Rejmanek M and Richardson DM (1996) What attributes make some plant species more invasive? Ecology 77:1651–1661
Resetarits WJ (2000) You can take it with you: colonization success and population structure of female-and pair-founded populations of Gambusia a.nis. Ecological Society of America Annual Meeting, Snowbird, Utah (Abstr)
Richardson DM, Pysek P, Rejmanek M, Barbour MG, Panet-ta FD and West CJ (2000) Naturalization and invasion of alien plants: concepts and definitions. Diversity and Distributions 6:93–107
Robbins LW, Hartman GD and Smith MH (1987) Dispersal, reproductive strategies, and the maintenance of genetic variability in mosquito fish (Gambusia a.nis ).Copeia 1987: 156–164
Rosen DE and Bailey RM (1963) The poeciliid fishes (Cyprin-odontiformes),their structure, zoogeography, and systematics.Bulletin of the American Museum of Natural History 126:1–176
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O' Neil P, Parker IM, Thomson JN and Weller SG (2001) The population biology of invasive species. Annual Review of Ecology and Systematics 32: 305–332
Scribner KT (1993) Hybrid zone dynamics are influenced by genotype-specific variation in life-history traits: experimental evidence from hybridizing Gambusia species. Evolution 47:632–646
Scribner KT, Wooten MC, Smith MH, Kennedy PK and Rhodes OE (1992) Variation in life history and genetic traits of Hawaiian mosquito fish populations.Journal of Evolutionary Biology 5:267–288
Seferta A, Guay P-J, Marzinotto E and Lefebvre L (2001) Learning differences between feral pigeons and zenaida doves:the role of neophobia and human proximity. Ethology 107:281–293
Sol D, Timmermans S and Lefebvre L (2002) Behavioral.exibility and invasion success in birds. Animal Behaviour 63: 495–502
Swingland IR (1983) Intraspeci c differences in movement.In: Swingland IR and Greenwood PJ (eds) The Ecology of Animal Movement, 102–115. Clarendon Press, Oxford,UK
Tilman D (1994) Competition and biodiversity in spatially structured habitats. Ecology 75:2–16
Vermeij GJ (1996) An agenda for invasion biology. Biological Conservation 78:3–9
Walsh RN and Cummins RA (1976) The open-eld test:a critical review. Psychological Bulletin 83:482–504
Webb C and Joss J (1997) Does predation by the fish Gambusia holbrooki contribute to declining frog populations? Australian Zoologist 30:316–324
Welcomme RL (1992) A history of international introductions of inland aquatic species. ICES Marine Science Symposium 194:3–14
Williamson M (1999)Invasions.Ecography 22: 5–12
Wilson DS, Coleman K, Clark AB and Biederman L (1993) Shy-bold continuum in pumpkinseed sun fish (Lepomis gib-bosus ):an ecological study of a psychological trait. Journal of Comparative Psychology 107:250–260
Winkler P (1975) Thermal tolerance of Gambusia a.nis (Tele-ostei:Poeciliidae)from a warm Spring. I. Field tolerance under natural spring conditions. Physiological Zoology 48: 367–377
Wooten MC, Scribner KT and Smith MH (1988) Genetic variability and systematics of Gambusia in the southeastern United States. Copeia 1988:283–289
Zane L, Nelson WS, Jones AG and Avise JC (1999) Microsatellite assessment of multiple paternity in natural populations of a live-bearing fish, Gambusia holbrooki. Journal of Evolutionary Biology 12:61–69
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rehage, J.S., Sih, A. Dispersal Behavior, Boldness, and the Link to Invasiveness: A Comparison of Four Gambusia Species. Biological Invasions 6, 379–391 (2004). https://doi.org/10.1023/B:BINV.0000034618.93140.a5
Issue Date:
DOI: https://doi.org/10.1023/B:BINV.0000034618.93140.a5