Abstract
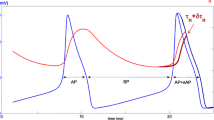
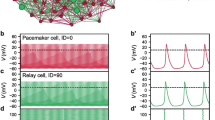
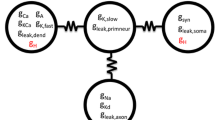
We have constructed mathematical models of the electrical activity of two hypothalamic supraoptic neuro-secretory cell-types, and we support our models with new calcium imaging and in vitro electrophysiological data. These cells are neurones that project to the pituitary gland and secrete either of two hormones, oxytocin or vasopressin, into the blood from their axonal terminals. Oxytocin-secreting and vasopressin-secreting cells are closely related and physically they differ only subtly, however when physiologically stressed their discharge patterns are dramatically distinct. We first show how each potassium current contributes to the action-potentials and after-potentials observed in these cells, and we show how these after-potentials are correlated to intra-cellular calcium elevations. We then show how these currents regulate the excitability of these cells and consequently shape their discharge pattern.
Similar content being viewed by others
References
Adams PR, Constanti A, Brown DA, Clark RB (1982a) Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature, 296(April): 746-749.
Adams PR, Brown DA, Constanti A (1982b) M-currents and other potassium currents in bullfrog sympathetic neurons. Journal of Physiology (London) 330: 537-572.
Andrew RD (1987) Endogenous bursting by rat supraoptic neuroendocrine cells is calcium dependent. Journal of Physiology (London) 384: 451-465.
Andrew RD, Dudek FE (1983) Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science 221(4615): 1050-1052.
Andrew RD, Dudek FE (1984a) Analysis of intracellularly recorded phasic bursting by mammalian neuroendocrine cells. Journal of Neurophysiology 51(3): 552-566.
Andrew RD, Dudek FE (1984b) Intrinsic inhibition in magnocellular neuroendocrine cells of rat hypothalamus. Journal of Physiology (London) 353: 171-185.
Aradi I, Holmes WR (1999) Role of multiple calcium and calcium-dependent conductances in regulation of hippocampal dentate granule cell excitability. Journal of Computational Neuroscience 6: 215-235.
Arai R, Jacobowitz DM, Nagatsu I (1996) Calretinin is differentially localized in magnocellular oxytocin neurons of the rat hypothalamus. A double-labeling immunofluorescence study. Brain Research 735(1): 154-158.
Arai R, Jacobowitz DM, Hida T (1999) Calbindin D28k and calretinin in oxytocin and vasopressin neurons of the rat supraoptic nucleus: A triple-labeling immunofluorescence study. Cell and Tissue Research 298(1): 11-19.
Armstrong WE, Smith BN (1990) Tuberal supraoptic neurons II: Electrotonic properties. Neuroscience 38(2): 485-494.
Armstrong WE, Smith BN, Tian M(1994) Electrophysiological characteristics of immunochemically identified rat oxytocin and vasopressin neurons in vitro. Journal of Physiology (London) 475(1): 115-128.
Bains JS, Ferguson AV (1999) Activation of N-methyl-D-aspartate receptors evokes calcium spikes in the dendrites of rat hypothalamic paraventricular nucleus neurons. Neuroscience 90(3): 885-891.
Blackwell KT (2002) The effect of intensity and duration on the light-induced sodium and potassium currents in the Hermissenda type B photoreceptor. Journal of Neuroscience 22: 4217-4228.
Borg-Graham L (1987) Modelling the somatic electrical behavior of hippocampal pyramidal neurons. M.Phil. thesis, Massachusetts Institute of Technology, Department of Electrical Engineering and Computer Science. Also appears as MIT AI Lab TR 1161.
Borg-Graham LJ (1999) Interpretations of data and mechanisms for hippocampal pyramidal cell models. In: PS Ulinski, EG Jones, A Peters, eds. Models of Cortical Circuits. Cerebral Cortex, New York: Plenum Press, Vol. 13, Chap. 2, pp. 19-138.
Bourque CW (1986) Calcium-dependent spike after-current induces burst firing in magnocellular neurosecretory-cells. Neuroscience Letters 70(2): 204-209.
Bourque CW (1988) Transient calcium-dependent potassium current in magnocellular neurosecretory cells of the rat supraoptic nucleus. Journal of Physiology (London) 397: 331-347.
Bourque CW, Brown DA (1987) Apamin and d-tubocurarine block the after-hyperpolarization of rat supraoptic neurosecretory neurons. Neuroscience Letters 82(2): 185-190.
Bourque, CW, Renaud LP (1988) Activity dependence of action potential duration in neurosecretory cells. In: G Leng ed. Pulsatility in Neuroendocrine Systems. CRC Press, Inc., Boca Raton, FL: Chap. 11, pp. 197-203.
Bourque CW, Randle JCR, Renaud LP (1985) Calcium-dependent potassium conductance in rat supraoptic nucleus neurosecretory neurons. Journal of Neurophysiology 54(6): 1375-1382.
Bourque CW, Kirkpatrick K, Jarvis CR (1998) Extrinsic modulation of spike afterpotentials in rat hypothalamoneurohypophysial neurons. Cellular and Molecular Neurobiology 18(1): 3-12.
Brimble MJ, Dyball REJ (1977) Characterization of oxytocin-and vasopressin-secreting neurones in the supraoptic nucleus to osmotic stimulation. Journal of Physiology (London) 271: 253-271.
Brown DA (2000) Neurobiology: The acid test for resting potassium channels. Current Biology 10: R456-R459.
Calvin WH (1975) Generation of spike trains in CNS neurons. Brain Research 84(1): 1-22.
Cazalis M, Dayanithi G, Nordmann JJ (1985) The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. Journal of Physiology (London) 369: 45-60.
Chad JE, Eckert R (1984) Calcium domains associated with individual channels can account for anomalous voltage relations of Ca-dependent responses. Biophysical Journal 45(5): 993-999.
Cobbett P, Mason WT (1987) Whole cell voltage clamp recordings from cultured neurons of the supraoptic area of neonatal rat hypothalamus. Brain Research 409(1): 175-180.
Cobbett P, Legendre P, Mason WT(1989) Characterization of 3 types of potassium current in cultured neurons of rat supraoptic nucleus area. Journal of Physiology (London) 410: 443-462.
Dopico AM, Widmer H, Wang G, Lemos JR, Treistman SN (1999) Rat supraoptic magnocellular neurones show distinct large conductance, Ca2+-activated K+ channel subtypes in cell bodies versus nerve endings. Journal of Physiology (London) 519(1): 101–114.
Ermentrout B (2002) Simulating, Analyzing, and Animating Dynamical Systems: A Guide to XPPAUT for Researchers and Students. SIAM, Philadelphia, PA.
Fisher TE, Bourque CW (1995) Voltage-gated calcium currents in the magnocellular neurosecretory cells of the rat supraoptic nucleus. Journal of Physiology (London) 486(3): 571–580.
Fisher TE, Bourque CW (1998) Properties of the transient K+ current in acutely isolated supraoptic neurons from adult rat. Advances in Experimental Medicine and Biology 449: 97-106.
Fisher TE, Voisin DL, Bourque CW (1998) Density of transient K+ current influences excitability in acutely isolated vasopressin and oxytocin neurones of rat hypothalamus. Journal of Physiology (London) 511(2): 423-432.
Foehring RC, Armstrong WE (1996) Pharmacological dissection of high-voltage-activated Ca2+ current types in acutely dissociated rat supraoptic magnocellular neurons. Journal of Neurophysiology 76(2): 977-983.
Ghamari-Langroudi M, Bourque CW (2000) Excitatory role of the hyperpolarization-activated inward current in phasic and tonic firing of rat supraoptic neurons. Journal of Neuroscience 20: 4855–4863.
Ghamari-Langroudi M, Bourque CW (2002) Flufenamic acid blocks depolarizing afterpotentials and phasic firing in rat supraoptic neurones. Journal of Physiology (London) 545(2): 537–542.
Golowasch J, Goldman MS, Abbott LF, Marder E (2002) Failure of averaging in the construction of a conductance-based neuron model. Journal of Neurophysiology 87(2): 1129-1131.
Greene CC, Schwindt PC, Crill WE (1994) Properties and ionic mechanisms of a metabotropic glutamate receptor-mediated slow afterdepolarization in neocortical neurons. Journal of Neurophysiology 72(2): 693-704.
Greffrath W, Martin E, Reuss S, Boehmer G (1998) Components of after-hyperpolarization in magnocellular neurons of the rat supraoptic nucleus in vitro. Journal of Physiology (London) 513(2): 493-506.
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry 260(6): 3440-3450.
Han J, Gnatenco C, Sladek CD, Kim D (2003) Background and tandem-pore potassium channels in magnocellular neurosecretory cells of the rat supraoptic nucleus. Journal of Physiology 546: 625–639.
Higuchi T, Honda K, Takano S, Negoro H (1988) Role of the supraoptic nucleus in regulation of parturition and milk ejection revisited. Journal of Endocrinology 116(2): 225-230.
Hille B (2001) Ionic Channels of Excitable Membranes. Third edn. Sunderland, Massachusetts: Sinauer Associates Inc.
Jahromi BS, Zhang L, Carlen PL, Pennefather P (1999) Differential time-course of slow afterhyperpolarizations and associated Ca2+ transients in rat CA1 pyramidal neurons: further dissociation by Ca2+ buffer. Neuroscience 88(3): 719-726.
Joux N, Chevaleyre V, Alonso G, Boissin-Agasse L, Moos FC, Desarménien MG, Hussy N (2001) High voltage-activated Ca2+ currents in rat supraoptic neurones: biophysical properties and expression of the various channel α1 subunits. Journal of Neuroendocrinology 13(7): 638-649.
Kirkpatrick K (1997) Functional role and modulation of a calciumactivated potassium current in rat supraoptic neurons in vitro. Ph.D. thesis, Department of Neurology and Neurosurgery, McGill University, Montreal.
Kirkpatrick K, Bourque CW (1995) Effects of neurotensin on rat supraoptic nucleus neurons in vitro. Journal of Physiology (London) 482(2): 373-381.
Kirkpatrick K, Bourque CW (1996) Activity dependence and functional role of the apamin-sensitive K+ current in rat supraoptic neurones in vitro. Journal of Physiology (London) 494(2): 389–398.
Komori Y, Dayanithi G, Sasaki N, Ishii1 M, Ueta Y, Shibuya I (2001) Analysis of the Ca2+ clearance mechanism of rat supraoptic neurons. Society for Neuroscience Abstracts 27(178.2).
Lambert RC, Dayanithi G, Moos FC, Richard P (1994) A rise in the intracellular Ca2+ concentration of isolated rat supraoptic cells in response to oxytocin. Journal of Physiology (London) 478(2): 275-287.
Lancaster B (1991) Isolation of potassium currents. In: J Chad, H Wheal, eds. Cellular Neurobiology: A Practical Approach. Oxford University Press, New York, Chap. 6, pp. 97-119.
Lancaster B, Zucker RS (1994) Photolytic manipulation of Ca2+ and the time-course of slow, Ca2+-activated K+ current in rat hippocampal-neurons. Journal of Physiology (London) 475: 229–239.
Lasser-Ross N, Miyakawa H, Lev-Ram V, Young SR, Ross WN (1991) High time resolution fluorescence imaging with a CCD camera. Journal of Neuroscience Methods 36(2/3): 253–261.
Lasser-Ross N, Ross WN, Yarom Y (1997) Activity-dependent [Ca2+]i changes in guinea pig vagal motoneurons: Relationship to the slow afterhyperpolarization. Journal of Neurophysiology 78(2): 825-834.
Leng G, Brown D, Murphy NP (1995) Patterning of electrical activity in magnocellular neurones. In: T Saito, K Kurokawa, S Yoshida, eds. Neurohypophysis, Recent Progress of Vasopressin and Oxytocin Research. Proceedings of the 1st Joint World Congress of Neurohypophysis and Vasopressin, Nasu, Tochigi, Japan. Elsevier Science B.V., Amsterdam, pp. 225-235.
Li ZH, Hatton GI (1997a) Ca2+ release from internal stores: Role in generating depolarizing after-potentials in rat supraoptic neurones. Journal of Physiology (London) 498(2): 339-350.
Li ZH, Hatton GI (1997b) Reduced outward K+ conductances generate depolarizing after-potentials in rat supraoptic nucleus neurones. Journal of Physiology (London) 505(1): 95-106.
Li ZH, Decavel C, Hatton GI (1995) Calbindin-D28k–role in determining intrinsically generated firing patterns in rat supraoptic neurons. Journal of Physiology (London) 488(3): 601–608.
Luther JA, Tasker JG (2000) Voltage-gated currents distinguish parvocellular from magnocellular neurones in the rat hypothalamic paraventricular nucleus. Journal of Physiology (London) 523(1): 193-209.
McCormick DA, Huguenard JR (1992) A model of the electrophysiological properties of thalamocortical relay neurons. Journal of Neurophysiology 68(4): 1384-1400.
Meeker RB, McGinnis S, Greenwood RS, Hayward JN (1994) Increased hypothalamic glutamate receptors induced by water deprivation. Neuroendocrinology 60(5): 477-485.
Miyata S, Khan AM, Hatton GI (1998) Colocalization of calretinin and calbindin-D28k with oxytocin and vasopressin in rat supraoptic nucleus neurons: A quantitative study. Brain Research 785(1): 178-182.
Nagatomo T, Inenaga K, Yamashita H (1995) Transient outward current in adult-rat supraoptic neurons with slice patch-clamp technique: Inhibition by angiotensin-II. Journal of Physiology (London) 485(1): 87-96.
North RA (2000) Potassium-channel closure taken to TASK. Trends in Neurosciences 23(6): 234-235.
Oliet SHR, Bourque CW (1992) Properties of supraoptic magnocellular neurons isolated from the adult-rat. Journal of Physiology (London) 455: 291-306.
Plant RE (1978) The effects of calcium++ on bursting neurons: A modeling study. Biophysical Journal 21: 217-237.
Poulain DA, Brown D, Wakerley JB (1988) Statistical analysis of patterns of electrical activity in vasopressin-and oxytocinsecreting neurones. In: G Leng ed., Pulsatility in Neuroendocrine Systems. Boca Raton, Florida: CRC Press, Inc, Chap. 8, pp. 119–154.
Reid G, Flonta ML (2001) Cold transduction by inhibition of a background potassium conductance in rat primary sensory neurones. Neuroscience Letters 297: 171-174.
Roper P, Callaway JC, Armstrong WE (2001) Reconstructing phasic vasopressin cells. Society for Neuroscience Abstracts 27(178.3).
Roper P, Sherman A (2002) Mathematical analysis of firing pattern transitions in rat son magnocellular neurosecretory cells subject to osmotic stress. Society for Neuroscience Abstracts 28(273.3).
Roper P, Callaway J, Armstrong W (2003) Burst Initiation and Termination in Phasic Vasopressin Cells of the Rat SON: A Combined Mathematical, Electrical and Calcium Fluorescence Study. The Journal of Neuroscience, Submitted.
Sah P (1992) Role of calcium influx and buffering in the kinetics of a Ca2+-activated K+ current in rat vagal motoneurons. Journal of Neurophysiology 68(6): 2237-2247.
Schrader LA, Tasker JG (1997) Modulation of multiple potassium currents by metabotropic glutamate receptors in neurons of the hypothalamic supraoptic nucleus. Journal of Neurophysiology 78(6): 3428-3437.
Selyanko AA, Sim JA (1998) Ca2+-inhibited non-inactivating K+ channels in cultured rat hippocampal pyramidal neurones. Journal of Physiology (London) 510(1): 71-91.
Shao LR, Halvorsrud R, Borg-Graham L, Storm JF (1999) The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. Journal of Physiology (London) 521(1): 135-146.
Simon SM, Llinas RR (1985) Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophysical Journal 48(3): 485-498.
Stern JE, Armstrong WE (1995) Electrophysiological differences between oxytocin and vasopressin neurons recorded from female rats in vitro. Journal of Physiology (London) 488(3): 701-708.
Stern JE, Armstrong WE (1996) Changes in the electrical properties of supraoptic nucleus oxytocin and vasopressin neurons during lactation. Journal of Neuroscience 16(16): 4861-4871.
Stern JE, Armstrong WE (1997) Sustained outward rectification of oxytocinergic neurones in the rat supraoptic nucleus: Ionic dependence and pharmacology. Journal of Physiology (London) 500(2): 497-508.
Stern JE, Galarreta M, Foehring RC, Hestrin S, Armstrong WE (1999) Differences in the properties of ionotropic glutamate synaptic currents in oxytocin and vasopressin neuroendocrine neurons. Journal of Neuroscience 19(9): 3367-3375.
Storm JF (1987) Action-potential repolarization and a fast afterhyperpolarization in rat hippocampal pyramidal cells. Journal of Physiology (London) 385: 733-759.
Storm JF (1993) Functional diversity of K+ currents in hippocampal pyramidal neurons. Seminars in the Neurosciences 5: 79-92.
Teruyama R, Armstrong WE (2002) Changes in the active membrane properties of rat supraoptic neurons during pregnancy and lactation. Journal of Neuroendocrinology 14: 1-17.
Van Goor F, Li YX, Stojilkovic SS (2001) Paradoxical role of large-conductance calcium-activated K+ (BK) channels in controlling action potential-driven Ca2+ entry in anterior pituitary cells. Journal of Neuroscience 21: 5902-5915.
Vergara C, Latorre R, Marrion, NV, Adelman JP 1998. Calcium-activated potassium channels. Current Opinion in Neurobiology 8(3): 321-329.
Voisin DL, Bourque CW (2002) Integration of sodium and osmosensory signals in vasopressin neurons. Trends in Neurosciences 25(4): 199-205.
Wakerley JB, Lincoln DW (1973) The milk-ejection reflex of the rat: A 20-to 40-fold acceleration in the firing of paraventricular neurones during oxytocin release. Journal of Endocrinology 57: 477-493.
Wakerley JB, Poulain DA, Brown D (1978) Comparison of firing patterns in oxytocin-and vasopressin-releasing neurones during progressive dehydration. Brain Research 148: 425-440.
Warman EN, Durand DM, Yuen GLF (1994) Reconstruction of hippocampal CA1 pyramidal cell electrophysiology by computer-simulation. Journal of Neurophysiology 71: 2033–2045.
Wilson CJ, Callaway JC (2000) Coupled oscillator model of the dopaminergic neuron of the substantia nigra. Journal of Neurophysiology 83(5): 3084-3100.
Yuen GLF, Durand D (1991) Reconstruction of hippocampal granule cell electrophysiology by computer-simulation. Neuroscience 41(2/3): 411-423.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Roper, P., Callaway, J., Shevchenko, T. et al. AHP's, HAP's and DAP's: How Potassium Currents Regulate the Excitability of Rat Supraoptic Neurones. J Comput Neurosci 15, 367–389 (2003). https://doi.org/10.1023/A:1027424128972
Issue Date:
DOI: https://doi.org/10.1023/A:1027424128972