Abstract
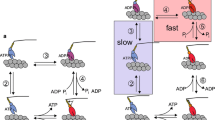
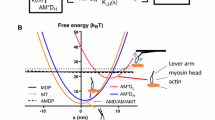
Our objective is to propose an overview of the usefulness of skeletal myofibril as an experimental system for studying mechanochemical coupling of skeletal muscles and myosin ATPase activity. The myofibril is a true functional mini-muscle that is able to contract in the presence of ATP. It also contains the machinery necessary for the calcium sensitivity of the contraction. In the absence of calcium, myofibrillar ATPase activity is basal, no shortening occurs and no active force is developed. In the presence of calcium, myofibrillar ATPase is activated and myofibrils either shorten with no external load (native myofibrils) or contract isometrically (cross-linked myofibrils). With this organised system, both chemical and mechanical studies can be carried out. For a decade, our laboratory has been using rabbit psoas myofibrils for exploring myosin ATPase activity. The first challenge was to successfully apply rapid kinetic approaches, such as rapid-flow-quench, to this organised system. Another challenge was to work with myofibrils in cryoenzymic conditions, i.e. in the presence of organic solvents and at sub-zero temperatures. In this overview, we highlight differences between the myosin ATPase in organised systems (myofibrils or fibres) and that of contractile proteins in solution (S1 or actoS1) that we observed using these approaches. We discuss the importance of these differences in terms of mechanochemical coupling. It is concluded that great care should be taken when extrapolating mechanochemical properties of the contractile proteins in solution to the whole muscle.
Similar content being viewed by others
References
Bagshaw CR and Trentham DR (1974) The characterization of myosin-product complexes and of product-release steps during the magnesium ion-dependent adenosine triphosphatase reaction. Biochem J 141: 331–349.
Barman TE and Travers F (1985) The rapid-flow-quench method in the study of fast reactions in biochemistry: extension to subzero conditions. Methods Biochem Anal 31: 1–59.
Bartoo ML, Popov VI, Fearn LA and Pollack GH (1993) Active tension generation in isolated skeletal myofibrils. J Muscle Res Cell Motil 14: 498–510.
Biosca JA, Travers F, Barman TE, Bertrand R, Audemard E and Kassab R (1985) Transient kinetics of adenosine 5′-triphosphate hydrolysis by covalently cross-linked actomyosin complex in water and 40% ethylene glycol by the rapid flow quench method. Biochemistry 24: 3814–3820.
Biosca JA, Travers F, Hillaire D and Barman TE (1984) Cryoenzymic studies on myosin subfragment 1: perturbation of an enzyme reaction by temperature and solvent. Biochemistry 23: 1947–1955.
Brenner B (1988) Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci USA 85: 3265–3269.
Brune M, Hunter JL, Corrie JE and Webb MR (1994) Direct, real-time measurement of rapid inorganic phosphate release using a novel fluorescent probe and its application to actomyosin subfragment 1 ATPase. Biochemistry 33: 8262–8271.
Colomo F, Piroddi N, Poggesi C, te Kronnie G and Tesi C (1997) Active and passive forces of isolated myofibrils from cardiac and fast skeletal muscle of the frog. J Physiol 500: 535–548.
Dantzig JA, Goldman YE, Luttmann ML, Trentham DR and Woodward SKA (1989) Binding of caged ATP diastereoisomers to rigor cross-bridges in glycerol-extracted fibres of rabbit psoas muscle. J Physiol 418: 61P.
Douzou P and Petsko GA (1984) Proteins at work: ‘stop-action’ pictures at subzero temperatures. Adv Protein Chem 36: 245–361.
Fenn WO (1924) The relation between the work performed and the energy liberated in muscular contraction. J Physiol 58: 373–395.
Ferenczi MA (1986) Phosphate burst in permeable muscle fibers of the rabbit. Biophys J 50: 471–477.
Ferenczi MA, He ZH, Chillingworth RK, Brune M, Corrie JE, Trentham DR and Webb MR (1995) A new method for the timeresolved measurement of phosphate release in permeabilized muscle fibers. Biophys J 68: 191S–192S (discussion 192S–193S).
Ferenczi MA, Homsher E and Trentham DR (1984) The kinetics of magnesium adenosine triphosphate cleavage in skinned muscle fibres of the rabbit. J Physiol 352: 575–599.
Friedman AL and Goldman YE (1996) Mechanical characterization of skeletal muscle myofibrils. Biophys J 71: 2774–2785.
Geeves MA (1991) The dynamics of actin and myosin association and the crossbridge model of muscle contraction. Biochem J 274: 1–14.
Geeves MA, Goody RS and Gutfreund H (1984) Kinetics of acto-S1 interaction as a guide to a model for the crossbridge cycle. J Muscle Res Cell Motil 5: 351–361.
Goldman YE, Hibberd MG, McCray JA and Trentham DR (1982) Relaxation of muscle fibres by photolysis of caged ATP. Nature 300: 701–705.
He ZH, Chillingworth RK, Brune M, Corrie JE, Trentham DR, Webb MR and Ferenczi MA (1997) ATPase kinetics on activation of rabbit and frog permeabilized isometric muscle fibres: a real time phosphate assay. J Physiol 501: 125–148.
He ZH, Ferenczi MA, Brune M, Trentham DR, Webb MR, Somlyo AP and Somlyo AV (1998) Time-resolved measurements of phosphate release by cycling cross-bridges in portal vein smooth muscle. Biophys J 75: 3031–3040.
Herrmann C, Houadjeto M, Travers F and Barman T (1992) Early steps of the Mg2+-ATPase of relaxed myofibrils. A comparison with Ca2+-activated myofibrils and myosin subfragment 1. Biochemistry 31: 8036–8042.
Herrmann C, Lionne C, Travers F and Barman T (1994) Correlation of ActoS1, myofibrillar, and muscle fiber ATPases. Biochemistry 33: 4148–4154.
Herrmann C, Sleep J, Chaussepied P, Travers F and Barman T (1993) A structural and kinetic study on myofibrils prevented from shortening by chemical cross-linking. Biochemistry 32: 7255–7263.
Hibberd MG and Trentham DR (1986) Relationships between chemical and mechanical events during muscular contraction. Annu Rev Biophys Biophys Chem 15: 119–161.
Homsher E and Millar NC (1990) Caged compounds and striated muscle contraction. Annu Rev Physiol 52: 875–896.
Knight PJ and Trinick JA (1982) Preparation of myofibrils. Methods Enzymol 85: 9–12.
Lionne C, Brune M, Webb MR, Travers F and Barman T (1995) Time resolved measurements show that phosphate release is the rate limiting step on myofibrillar ATPases. FEBS Lett 364: 59–62.
Lionne C, Iorga B, Candau R, Piroddi N, Webb MR, Belus A, Travers F and Barman T (2002) Evidence that phosphate release is the ratelimiting step on the overall ATPase of psoas myofibrils prevented from shortening by chemical cross-linking. Biochemistry 41: 13297–13308.
Lionne C, Stehle R, Travers F and Barman T (1999) Cryoenzymic studies on an organized system: myofibrillar ATPases and shortening. Biochemistry 38: 8512–8520.
Lionne C, Travers F and Barman T (1996) Mechanochemical coupling in muscle: attempts to measure simultaneously shortening and ATPase rates in myofibrils. Biophys J 70: 887–895.
Ma YZ and Taylor EW (1994) Kinetic mechanism of myofibril ATPase. Biophys J 66: 1542–1553.
Malik MN and Martonosi A (1972) The regulation of the rate of ATP hydrolysis by H-meromyosin. Arch Biochem Biophys 152: 243–257.
Mornet D, Bertrand RU, Pantel P, Audemard E and Kassab R (1981) Proteolytic approach to structure and function of actin recognition site in myosin heads. Biochemistry 20: 2110–2220.
Ohno T and Kodama T (1991) Kinetics of adenosine triphosphate hydrolysis by shortening myofibrils from rabbit psoas muscle. J Physiol 441: 685–702.
Rosenfeld SS and Taylor EW (1984) The ATPase mechanism of skeletal and smooth muscle acto-subfragment 1. J Biol Chem 259: 11908–11919.
Sleep J, Herrmann C, Barman T and Travers F (1994) Inhibition of ATP binding to myofibrils and acto-myosin subfragment 1 by caged ATP. Biochemistry 33: 6038–6042.
Stehle R, Lionne C, Travers F and Barman T (1998) Probing the coupling of Ca2+ and rigor activation of rabbit psoas myofibrillar ATPase with ethylene glycol. J Muscle Res Cell Motil 19: 381–392.
Stehle R, Lionne C, Travers F and Barman T (2000) Kinetics of the initial steps of rabbit psoas myofibrillar ATPases studied by tryptophan and pyrene fluorescence stopped-flow and rapid flow-quench. Evidence that cross-bridge detachment is slower than ATP binding. Biochemistry 39: 7508–7520.
Stehle R, Kruger M and Pfitzer G (2002) Force kinetics and individual sarcomere dynamics in cardiac myofibrils after rapid Ca2+ changes. Biophys J 83: 2152–2161.
Taylor EW (1979) Mechanism of actomyosin ATPase and the problem of muscle contraction. CRC Crit Rev Biochem 6: 103–164.
Tesi C, Barman T and Travers F (1990a) Is a four-state model sufficient to describe actomyosin ATPase? FEBS Lett 260: 229–232.
Tesi C, Travers F and Barman T (1990b) Cryoenzymic studies on actomyosin ATPase. Evidence that the degree of saturation of actin with myosin subfragment 1 affects the kinetics of the binding of ATP. Biochemistry 29: 1846–1852.
Tesi C, Colomo F, Nencini S, Piroddi N and Poggesi C (2000) The effect of inorganic phosphate on force generation in single myofibrils from rabbit skeletal muscle. Biophys J 78: 3081–3092.
Tesi C, Piroddi N, Colomo F and Poggesi C (2002) Relaxation kinetics following sudden Ca2+ reduction in single myofibrils from skeletal muscle. Biophys J 83: 2142–2151.
Travers F and Barman T (1995) Cryoenzymology: how to practice kinetic and structural studies. Biochimie 77: 937–948.
Trentham DR, Eccleston JF and Bagshaw CR (1976) Kinetic analysis of ATPase mechanisms. Q Rev Biophys 9: 217–281.
Webb MR, Hibberd MG, Goldman YE and Trentham DR (1986) Oxygen exchange between Pi in the medium and water during ATP hydrolysis mediated by skinned fibers from rabbit skeletal muscle. Evidence for Pi binding to a force-generating state. J Biol Chem 261: 15557–15564.
White HD (1985) Kinetics of tryptophan fluorescence enhancement in myofibrils during ATP hydrolysis. J Biol Chem 260: 982–986.
White HD, Belknap B and Webb MR (1997) Kinetics of nucleoside triphosphate cleavage and phosphate release steps by associated rabbit skeletal actomyosin, measured using a novel fluorescent probe for phosphate. Biochemistry 36: 11828–11836.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lionne, C., Iorga, B., Candau, R. et al. Why choose myofibrils to study muscle myosin ATPase?. J Muscle Res Cell Motil 24, 139–148 (2003). https://doi.org/10.1023/A:1026045328949
Issue Date:
DOI: https://doi.org/10.1023/A:1026045328949