Abstract
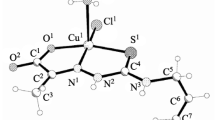
The coordination behaviour of the title ligand, 5-methyl-3-formylpyrazole N(4)-benzyl-N(4)-methylthiosemicarbazone(HMPz4BM), is reported with solid state isolation of copper(II) complexes, [Cu(HMPz4BM)X2] (X = Cl, Br, NO3, ClO4 and BF4) which have been spectroscopically and structurally characterised. I.r. data for the free ligand and its Cu(II) complexes indicate that HMPz4BM exhibits a neutral NNS tridentate function via the pyrazolyl nitrogen(tertiary), azomethine nitrogen and thione sulphur. Electronic spectral data are suggestive of a square pyramidal environment for the seemingly pentacoordinated Cu(II) species. E.s.r parameters (RT and LNT) of the reported copper(II) complexes are indicative of a dxx2–y2 ground state for the reported species. Cyclic voltammograms of Cu(II) complexes show a quasireversible CuII/CuIII couple and also an irreversible CuII/CuI couple. X-ray crystallography of a representative species, [Cu(HMPz4BM)(NO3)2] (C2/c, monoclinic ), has unambiguously documented the conjectural findings from i.r. data that coordinating sites of the title ligand are pyrazolyl (tertiary)nitrogen, azomethine nitrogen and the thione sulphur (NNS); and the oxygen of one of the nitrate ions has occupied the basal plane; the fifth coordination position has been occupied by the oxygen of another nitrate ion in a square pyramidal geometry. The antibacterial properties of the ligand and its copper(II) complexes studied on microorganism, Staphylococcus aureus have pointed out that most of the complexes have higher activities than that of the free ligand.
Similar content being viewed by others
References
Liberta AE, West DX: Antifungal and antitumor activity of heterocyclic thiosemicarbazones and their metal complexes: Current status. Biometals 5: 121-126 + refs, 1992
Li J, Zheng LM, King I, Doyle TW, Chen SH: Synthesis and antitumor activities of potent inhibitors of ribonucleotide reductase: 3-amino-4-methylpyridine-2-carboxaldehyde thiosemicarbazone (3-AP) and its water-soluble prodrugs. Curr Med Chem 8: 121-133, 2001
Klayman DL, Scovill JP, Bartosevich JF, Mason CJ: 2-Acetyl pyridine Agents. Thiosemicarbazones. 2 N4, N4-disubstituted derivatives as potential antimalarial agents. J Med Chem 22: 1367-1373, 1979
Teitz Y, Barko N, Abramoff M, Ronen D: Relationships between structure and antiretroviral activity of thiosemicarbazone derivatives. Chemotherapy 40: 195-200, 1994
West DX, Liberta AE, Padhye SB, Chikate RC, Sonawane PB, Kumbhar AS, Yerande RG: Thiosemicarbazone complexes of copper(II): Structural and biological studies. Coord Chem Rev 123: 49-71, 1993
West DX, Padhye SB, Sonawane PB: Structural and Physical Correlations in the Biological Properties of Transition Metal Heterocyclic Thiosemicarbazone and S-alkyldithiocarbazate Complexes. Struct Bonding 76: 1-50, 1991
Ali MK, Mirza AH, Iran MSH, Butcher RJ, Tarafdar MTH, Keat TB, Ali AM: Magnetic, spectroscopic and biological properties of copper (II) complexes of the tridentate ligand, α-N-methyl-S-methyl-β-N-(2-pyridyl) methylenedithiocarbazate(NNS) and the X-ray crystal structure of the [Cu(NNS)I2] complex. Trans Met Chem 27: 262-267, 2002
Amir M, Khan SA, Drabu S: Synthesis and antibacterial activity of 4-arylazo-1-substituted 3,5-diphenylpyrazoles. J Indian Chem Soc 79: 280-281, 2002
Saha NC, Seal A, Butcher RJ, Saha N: Synthesis and spectroscopic identification of cobalt(III) complexes with 5-methyl-3-formylpyrazole-N(4)-dipropylthiosemicarbazone(HMPzNPr2): X-ray crystal structure of [Co(MPzNPr2)2]NO3.H2O. J Indian Chem Soc 78: 601-605, 2001
Saha NC, Butcher RJ, Chaudhuri S, Saha N: Synthesis and spectroscopic studies of cobalt(III) complexes with 5-methyl-3-formylpyrazole-N(4)-diethylthiosemicarbazone(HMPzNEt2): X-ray crystallography of [Co(MPzNEt2)2]ClO4.2H2O (I) and [Co(MPzNEt2)2]BF4.2H2O (II). Polyhedron 21: 779-785, 2002
Saha NC, Saha A, Butcher RJ, Chaudhuri S, Saha N: Synthesis and structural characterization of new iron(III) complexes with biologically relevant pyrazolyl thiosemicarbazones. Inorg Chim Acta 339C: 348-354, 2002
Sau DK, Saha N, Butcher RJ, Chaudhuri S: Synthesis, spectroscopy and cyclic voltammetry of cobalt(III) complexes with 5-methyl-3-formylpyrazole N(4)-cyclohexylthiosemicarbazone (HMPz4Cy): X-ray crystal structure of [Co(MPz4Cy)2]Cl.2.75H2O. Trans Met Chem 28: 229, 2003
Saha N, Mukherjee N: Synthesis, characterization and coordinating properties of a new pyrazole derived Thiosemicarbazone, a potential antiviral agent: Co(III), Ni(II) and Cu(II) complexes of neutral and deprotonated 5(3)-methyl pyrazole-3(5)-aldehydothiosemicarbazone. Polyhedron 3: 1135-1140, 1984
Scovill JP: A facile synthesis of thiosemicarbazides and thiosemicarbazones by the transamination of a 4-methyl-4-phenyl-3-thiosemicarbazide. Phosphorus Sulfur Silicon 60: 15-19, 1991
Sheldrick GM: SHELX 97, Program for the Refinement of Crystal Structure. University of Göttingen, Göttingen, Germany, 1997
Cromer DT, Weber JT: International Tables for X-ray Crystallography, Vol. IV (Table 2.2A). The Keynoch Press, Birmingham, England, 1994
Drago RS: Physical Methods for Chemists. Saunders College Publishing, Florida, 1992, pp. 429-449
Saha N, Mukherjee N: Ligational behavior of a new pyrazole derived Thiosemicarbazones, a potential antiviral agent, cobalt(III), nickel(II) and copper(II) complexes of neutral and deprotonated 5(3)-methyl pyrazole-3(5)-aldehydo-4-phenylthiosemicarbazones. Synth React Inorg Met-Org Chem 14: 1151-1171, 1984
West DX, Beraldo H, Nassar AA, El-Saied FA, Ayad MI: Copper(II) complexes of 4-acetamido benzaldehyde N(4)-substituted thiosemicarbazones. Trans Met Chem 24: 421-424, 1999
Huang GS, Lai JK, Ueng CH, Su CC: Molecular and electronic structures of bis (dipicolylamine) copper(II) perchlorate and bis [2-(2-pyridyl ethyl) picolylamine] copper(II) perchlorate. Trans Met Chem 25: 84-92, 2000
West DX, Swearingen JK, El-Sawaf AK: Copper(II) complexes of 2-pyridine formamide N(4)-methylthiosemicarbazone. Trans Met Chem 25: 80-83, 2000
Tomlinson AAG, Hathaway BJ: The electronic properties and stereochemistry of the copper(II) Ion. Part II. The monoamine adducts of bisethylene diamine copper(II) complexes. J Chem Soc A: 1685-1688, 1968
Procter IM, Hathaway BJ, Nicholis P: The electronic properties and stereochemistry of the copper(II) ion. Part I. Bis (ethylene diamine) copper(II) complexes. J Chem Soc A: 1678-1684, 1968
Nohria L, Mathur R, Mathur P: Synthesis and characterization of copper(I) and copper(II) complexes with 1,5-bis (benzimidazol-2-yl)-3-thiapentane. Indian J Chem 38A: 256-261, 1999
Hathaway BJ, Billing DE: The electronic properties and stereochemistry of mono-nuclear complexes of the copper(II) ion. Coord Chem Rev 5: 143-207, 1970
Butcher RJ, West DX: Structure of the copper(II) complex of 2-acetylpyridine hexamethyleneiminylthiosemicarbazone, [Cu(Lhexim)Br]. Trans Met Chem 18: 449-452, 1993
Ali MA, Dey KK, Nazimuddin M, Smith FE, Butcher RJ, Jasinski JP, Jasinski JM: The preparation and characterization of some copper(II) complexes of the 6-methyl-2-formylpyridine thiosemicarbazone and the X-ray crystal structure of the chloro (6-methyl-2-formylpyridine thiosemicarbazone) copper(II) complex. Polyhedron 15: 3331-3339 + refs, 1996
Ainscough EW, Brodie AM, Ranford JD, Waters JM: Reaction of nitrogen and sulphur donor ligands with the antitumour complex [{CuL (MeCO2)}2] (HL = 2-formylpyridine thiosemicarbazone) and the single-crystal X-ray structure of [CuL(bipy)]ClO4 (bipy = 2,2ℜ-bipyridyl). J Chem Soc Dalton Trans: 1737-1742, 1991
Adhikari N, Chaudhuri S, Butcher RJ, Saha N: Synthesis and spectroscopic characterization of copper(II) complexes with 5-methyl-1-(2′-pyridyl) pyrazole-3-carboxamide (MPyPzCA): X-ray crystal structure of [Cu(MPyPzCA)2(H2O)](ClO4)2. Polyhedron 18: 1323-1328, 1999
Franco E, López-Torres E, Mendiola MA, Sevilla MT: Synthesis, spectroscopic and cyclic voltammetry studies of copper(II) complexes with open chain, cyclic and a new macrocyclic thiosemicarbazones. Polyhedron 19: 441-451, 2000
Kumbhar AS, Padhye SB, West DX, Liberta AE: Electrochemical studies of transition metal complexes of 2-acetyl pyridine thiosemicarbazones. Part 2. Correlation with spectral and antifungal properties of copper(II) complexes of 2-acetyl pyridine 3-azacyclothiosemicarbazones. Trans Met Chem 17: 247-249, 1992
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sau, D.K., Butcher, R.J., Chaudhuri, S. et al. Spectroscopic, structural and antibacterial properties of copper(II) complexes with bio-relevant 5-methyl-3-formylpyrazole N(4)-benzyl-N(4)-methylthiosemicarbazone. Mol Cell Biochem 253, 21–29 (2003). https://doi.org/10.1023/A:1026041032078
Issue Date:
DOI: https://doi.org/10.1023/A:1026041032078