Abstract
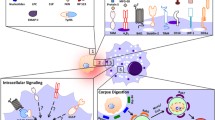
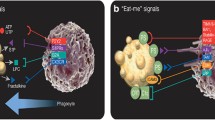
The termination of the apoptotic program occurs in most cases via recognition and clearance by phagocytes. Engulfed cells do not simply disappear from the midst of living tissues. Constituents of the corpse indeed survive the intracellular processing and are recycled to the membrane of the phagocyte. The presentation of yielded antigens to T cells is a central event in the induction and the maintenance of peripheral tolerance. Conversely, errors in this pathway contribute to the pathogenesis of systemic and organ specific autoimmune diseases. Here we discuss the available information on the events that follow active engulfment of dying cells, with attention to the events involved in vitro and in vivo in apoptotic cell processing. The outcome of the processing is the cross-priming or the functional inactivation of T cells that specifically recognise antigens contained in the cell corpse.
Similar content being viewed by others
References
Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature 2000; 407: 784-788.
Henson PM, Bratton DL, Fadok VA. The phosphatidylserine receptor: A crucial molecular switch? Nat Rev Mol Cell Biol 2001; 2: 627-633.
Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: Clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2002; 2: 965-975.
Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science 2000; 288: 2051- 2054.
Brown S, Heinisch I, Ross E, Shaw K, Buckley CD, Savill J. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature 2002; 418: 200-203.
Chimini G. Apoptosis: Repulsive encounters. Nature 2002; 418: 139-141.
Shiratsuchi A, Kaido M, Takizawa T, Nakanishi Y. Phosphatidylserine-mediated phagocytosis of influenza A virus-zinfected cells by mouse peritoneal macrophages. J Virol 2000; 74: 9240-9244.
Watanabe Y, Shiratsuchi A, Shimizu K, Takizawa T, Nakanishi Y. Role of phosphatidylserine exposure and sugar chain desialylation at the surface of influenza virus-infected cells in efficient phagocytosis by macrophages. J Biol Chem 2002; 277: 18222-18228.
Hengartner MO. Apoptosis: Corralling the corpses. Cell 2001; 104: 325-328.
Leverrier Y, Lorenzi R, Blundell MP, et al. Cutting edge: The Wiskott-Aldrich syndrome protein is required for efficient phagocytosis of apoptotic cells. J Immunol 2001; 166: 4831- 4834.
Leverrier Y and Ridley AJ. Requirement for Rho GTPases and PI 3-kinases during apoptotic cell phagocytosis by macrophages. Curr Biol 2001; 11: 195-199.
Vieira OV, Bucci C, Harrison RE, et al. Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase. Mol Cell Biol 2003; 23: 2501-2514.
Desjardins M. ER-mediated phagocytosis: A new membrane for new functions. Nat Rev Immunol 2003; 3: 280-291.
Henson PM, Bratton DL, Fadok VA. Apoptotic cell removal. Curr Biol 2001; 11: R795-R805.
Fadok VA, Bratton DL, Henson PM. Phagocyte receptors for apoptotic cells: Recognition, uptake, and consequences. J Clin Invest 2001; 108: 957-962.
Hoffmann PR, deCathelineau AM, Ogden CA, et al. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol 2001; 155: 649-659.
Bondanza A, Rovere-Querini P. Haematological autoimmunity in Systemic Lupus Erythematosus: Anti-phospholipid antibodies at the cross-road. In: Pandalai SG, ed. Recent Research Developments in Immunology. Trivandrum: Transworld Research Network, 2001; pp. 187-201.
Somersan S, Bhardwaj N. Tethering and tickling: A new role for the phosphatidylserine receptor. J Cell Biol 2001; 155: 501-504.
Albert ML, Kim JI, Birge RB. Alphavbeta5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat Cell Biol 2000; 2: 899-905.
Russo V, Zhou D, Sartirana C, et al. Acquisition of intact allogeneic human leukocyte antigen molecules by human dendritic cells. Blood 2000; 95: 3473-3477.
Platt N, da Silva RP, Gordon S. Recognizing death: The phagocytosis of apoptotic cells. Trends Cell Biol 1998; 8: 365- 372.
Gregory CD. CD14-dependent clearance of apoptotic cells: Relevance to the immune system. Curr Opin Immunol 2000; 12: 27-34.
Schlegel RA, Williamson P. Phosphatidylserine, a death knell. Cell Death Differ 2001; 8: 551-563.
Chimini G. Engulfing by lipids: A matter of taste? Cell Death Differ 2001; 8: 545-548.
Franc NC. Phagocytosis of apoptotic cells in mammals, caenorhabditis elegans and Drosophila melanogaster: Molecular mechanisms and physiological consequences. Front Biosci 2002; 7: d1298-d1313.
Vivers S, Dransfield I, Hart SP. Role of macrophage CD44 in the disposal of inflammatory cell corpses. Clin Sci (Lond) 2002; 103: 441-449.
Taylor PR, Carugati A, Fadok VA, et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med 2000; 192: 359-366.
Rovere P, Peri G, Fazzini F, et al. The long pentraxin PTX3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood 2000; 96: 4300- 4306.
Walport MJ. Complement. Second of two parts. N Engl J Med 2001; 344: 1140-1144.
Manfredi AA, Iannacone M, D'Auria F, Rovere-Querini P. The disposal of dying cells in living tissues. Apoptosis 2002; 7: 153-161.
Nauta AJ, Daha MR, Kooten C, Roos A. Recognition and clearance of apoptotic cells: A role for complement and pentraxins. Trends Immunol 2003; 24: 148-154.
Rovere-Querini P. Soluble factors that bind to dying cells control the outcome of corpse disposal: The role of pentraxins, connectins and Autoantibodies. In: Kalden JR, Herrmann M, eds. Apoptosis and Autoimmunity.Wiley-VCH, 2003: 79-95.
Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature 2002; 417: 182-187.
Monks J, Geske FJ, Lehman L, Fadok VA. Do inflammatory cells participate in mammary gland involution? J Mammary Gland Biol Neoplasia 2002; 7: 163-176.
Fazzini F, Peri G, Doni A, et al. PTX3 in small-vessel vasculitides: An independent indicator of disease activity produced at sites of inflammation. Arthritis Rheum 2001; 44: 2841-2850.
Hart SP, Jackson C, Kremmel LM, et al. Specific binding of an antigen-antibody complex to apoptotic human neutrophils. Am J Pathol 2003; 162: 1011-1018.
Conradt B. With a little help from your friends: Cells don't die alone. Nat Cell Biol 2002; 4: E139-E143.
Hoeppner DJ, Hengartner MO, Schnabel R. Engulfment genes cooperate with ced-3 to promote cell death in Caenorhabditis elegans. Nature 2001; 412: 202-206.
Reddien PW, Cameron S, Horvitz HR. Phagocytosis promotes programmed cell death in C. elegans. Nature 2001; 412: 198-202.
Green DR, Beere HM. Apoptosis. Mostly dead. Nature 2001; 412: 133-135.
Degen WG, Pruijn GJ, Raats JM, van Venrooij WJ. Caspasedependent cleavage of nucleic acids. Cell Death Differ 2000; 7: 616-627.
Clemens MJ, Bushell M, Jeffrey IW, Pain VM, Morley SJ. Translation initiation factor modifications and the regulation of protein synthesis in apoptotic cells. Cell Death Differ 2000; 7: 603-615.
Utz PJ, Anderson P. Life and death decisions: Regulation of apoptosis by proteolysis of signaling molecules. Cell Death Differ 2000; 7: 589-602.
Rovere P, Manfredi AA. Two or three (thousands) things to do before dying. Cell Death Differ 2000; 7: 587-588.
Vaughan AT, Betti CJ, Villalobos MJ. Surviving apoptosis. Apoptosis 2002; 7: 173-177.
Avallone B, Balsamo G, Trapani S, Marmo F. Apoptosis during chick inner ear development: Some observations by TEM and TUNEL techniques. Eur J Histochem 2002; 46: 53-59.
Hedge VL, Williams GT. Commitment to apoptosis induced by tumour necrosis factor-alpha is dependent on caspase activity. Apoptosis 2002; 7: 123-132.
Bonanno E, Tagliafierro G, Carla EC, et al. Synchronized onset of nuclear and cell surface modifications in U937 cells during apoptosis. Eur J Histochem 2002; 46: 61-74.
Fujimoto I, Pan J, Takizawa T, Nakanishi Y. Virus clearance through apoptosis-dependent phagocytosis of influenza Avirus-infected cells by macrophages. JVirol 2000; 74: 3399- 3403.
Holmgren L, Szeles A, Rajnavolgyi E, et al. Horizontal transfer of DNA by the uptake of apoptotic bodies. Blood 1999; 93: 3956-3963.
Spetz AL, Patterson BK, Lore K, Andersson J, Holmgren L. Functional gene transfer of HIV DNA by an HIV receptor-independent mechanism. J Immunol 1999; 163: 736- 742.
Bergsmedh A, Szeles A, Henriksson M, et al. Horizontal transfer of oncogenes by uptake of apoptotic bodies. Proc Natl Acad Sci USA 2001; 98: 6407-6411.
Bergsmedh A, Szeles A, Spetz AL, Holmgren L. Loss of the p21(Cip1/Waf1) cyclin kinase inhibitor results in propagation of horizontally transferred DNA. Cancer Res 2002; 62: 575- 579.
Ehrenreich BA, Cohn ZA. The fate of peptides pinocytosed by macrophages in vitro. J Exp Med 1969; 129: 227-245.
Cohn ZA, Ehrenreich BA. The uptake, storage, and intracellular hydrolysis of carbohydrates by macrophages. J Exp Med 1969; 129: 201-225.
Bellone M, Iezzi G, Rovere P, et al. Processing of engulfed apoptotic bodies yields T cell epitopes. J Immunol 1997; 159: 5391-5399.
Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 1998; 392: 86-89.
Inaba K, Turley S, Yamaide F, et al. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J Exp Med 1998; 188: 2163-2173.
Albert ML, Pearce SF, Francisco LM, et al. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med 1998; 188: 1359-1368.
Rovere P, Vallinoto C, Bondanza A, et al. Bystander apoptosis triggers dendritic cell maturation and antigen-presenting function. J Immunol 1998; 161: 4467-4471.
Bellone M. Apoptosis, cross-presentation, and the fate of the antigen specific immune response. Apoptosis 2000; 5: 307- 314.
Rovere P, Sabbadini MG, Vallinoto C, et al. Dendritic cell presentation of antigens from apoptotic cells in a proinflammatory context: Role of opsonizing anti-beta 2 glycoprotein I antibodies. Arthritis and Rheumatism 1999; 42: 1412-1420.
Harding CV, Ramachandra L, Wick MJ. Interaction of bacteria with antigen presenting cells: Influences on antigen presentation and antibacterial immunity. Curr Opin Immunol 2003; 15: 112-119.
Bouvier M. Accessory proteins and the assembly of human class I MHC molecules: A molecular and structural perspective. Mol Immunol 2003; 39: 697-706.
York IA, Mo AX, Lemerise K, et al. The cytosolic endopeptidase, thimet oligopeptidase, destroys antigenic peptides and limits the extent of MHC class I antigen presentation. Immunity 2003; 18: 429-440.
Schnurr M, Scholz C, Rothenfusser S, et al. Apoptotic pancreatic tumor cells are superior to cell lysates in promoting cross-priming of cytotoxic T cells and activate NK and gammadelta T cells. Cancer Res 2002; 62: 2347-2352.
Kovacsovics-Bankowski M, Rock KL. A phagosome-tocytosol pathway for exogenous antigens presented on MHC class I molecules. Science 1995; 267: 243-246.
Norbury CC, Hewlett LJ, Prescott AR, Shastri N, Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity 1995; 3: 783-791.
Sousa C, Germain RN. Major histocompatibility complex class I presentation of peptides derived from soluble exogenous antigen by a subset of cells engaged in phagocytosis. J Exp Med 1995; 182: 841-851.
Gromme M, Neefjes J. Antigen degradation or presentation by MHC class I molecules via classical and non-classical pathways. Mol Immunol 2002; 39: 181-202.
Gromme M, Uytdehaag FG, Janssen H, et al. RecyclingMHC class I molecules and endosomal peptide loading. Proc Natl Acad Sci USA 1999; 96: 10326-10331.
den Haan JM, Bevan MJ. Antigen presentation to CD8+ T cells: Cross-priming in infectious diseases. Curr Opin Immunol 2001; 13: 437-441.
Gagnon E, Duclos S, Rondeau C, et al. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell 2002; 110: 119-131.
Tirosh B, Furman MH, Tortorella D, Ploegh HL. Protein unfolding is not a prerequisite for endoplasmic reticulum-tocytosol dislocation. J Biol Chem 2003; 278: 6664-6672.
Wiertz EJ, Tortorella D, Bogyo M, et al. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature 1996; 384: 432-438.
Garin J, Diez R, Kieffer S, et al. The phagosome proteome: Insight into phagosome functions. J Cell Biol 2001; 152: 165- 180.
Gough MJ, Melcher AA, Ahmed A, et al. Macrophages orchestrate the immune response to tumor cell death. Cancer Res 2001; 61: 7240-7247.
Ramirez MC, Sigal LJ. Macrophages and dendritic cells use the cytosolic pathway to rapidly cross-present antigen from live, vaccinia-infected cells. J Immunol 2002; 169: 6733-6742.
Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 1998; 392: 86-89.
Kurts C, Miller JF, Subramaniam RM, Carbone FR, Heath WR. Major histocompatibility complex class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J Exp Med 1998; 188: 409-414.
Li M, Davey GM, Sutherland RM, et al. Cell-associated ovalbumin is cross-presented much more efficiently than soluble ovalbumin in vivo. J Immunol 2001; 166: 6099- 6103.
Larsson M, Fonteneau JF, Somersan S, et al. Efficiency of cross presentation of vaccinia virus-derived antigens by human dendritic cells. Eur J Immunol 2001; 31: 3432-3442.
Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol 2001; 19: 47-64.
Ronchetti A, Rovere P, Iezzi G, et al. Immunogenicity of apoptotic cells in vivo: Role of antigen load, antigen-presenting cells, and cytokines. J Immunol 1999; 163: 130-136.
Henry F, Boisteau O, Bretaudeau L, Lieubeau B, Meflah K, Gregoire M. Antigen-presenting cells that phagocytose apoptotic tumor-derived cells are potent tumor vaccines. Cancer Res 1999; 59: 3329-3332.
Bondanza A, Zimmermann VS, Dell'Antonio G, et al. Cutting edge: Dissociation between autoimmune response and clinical disease after vaccination with dendritic cells. J Immunol 2003; 170: 24-27.
Jung S, Unutmaz D, Wong P, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity 2002; 17: 211-220.
Rovere P, Sabbadini MG, Bondanza A, et al. Remnants of suicidal cells fostering systemic autoaggression: Apoptosis in the origin and maintenance of autoimmunity. Arthritis and Rheumatism 2000; 43: 1663-1672.
Rovere P, Manfredi AA, Vallinoto C, et al. Dendritic cells preferentially internalize apoptotic cells opsonized by antibeta2-glycoprotein I antibodies. J Autoimmun 1998; 11: 403- 411.
Akiyama K, Ebihara S, Yada A, et al. Targeting apoptotic tumor cells to FcgammaR provides efficient and versatile vaccination against tumors by dendritic cells. J Immunol 2003; 170: 1641-1648.
Bondanza A, Sabbadini MG, Pellegatta F, et al. Antibeta2 glycoprotein I antibodies prevent the De-activation of platelets and sustain their phagocytic clearance. J Autoimmun 2000; 15: 469-477.
Bondanza A, Manfredi AA, Zimmermann VS, et al. Antibeta2 glycoprotein I antibodies cause inflammation and recruit dendritic cells in platelet clearance. Thromb Haemost 2001; 86: 1257-1263.
den Haan JM, Bevan MJ. Constitutive versus activationdependent cross-presentation of immune complexes by CD8(+) and CD8(-) dendritic cells in vivo. J Exp Med 2002; 196: 817-827.
D'Agnillo P, Levine JS, Subang R, Rauch J. Prothrombin binds to the surface of apoptotic, but not viable, cells and serves as a target of lupus anticoagulant autoantibodies. J Immunol 2003; 170: 3408-3422.
Anderson HA, Maylock CA, Williams JA, Paweletz CP, Shu H, Shacter E. Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells. Nat Immunol 2003; 4: 87-91.
Stach CM, Turnay X, Voll RE, et al. Treatment with annexin V increases immunogenicity of apoptotic human T-cells in Balb/c mice. Cell Death Differ 2000; 7: 911-915.
Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002; 418: 191-195.
Rordorf C, Schnebli HP, Baltz ML, Tennent GA, Pepys MB. The acute-phase response in (NZB X NZW)F1 and MRL/l MICE. J Exp Med 1982; 156: 1268-1273.
Potter PK, Cortes-Hernandez J, Quartier P, Botto M, Walport MJ. Lupus-prone mice have an abnormal response to thioglycolate and an impaired clearance of apoptotic cells. J Immunol 2003; 170: 3223-3232.
Koh JS, Wang Z, Levine JS. Cytokine dysregulation induced by apoptotic cells is a shared characteristic of murine lupus. J Immunol 2000; 165: 4190-4201.
Bickerstaff MC, Botto M, Hutchinson WL, et al. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat Med 1999; 5: 694-697.
Garlanda C, Hirsch E, Bozza S, et al. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature 2002; 420: 182-186.
Botto M, Dell'Agnola C, Bygrave AE, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet 1998; 19: 56-59.
Vandivier RW, Ogden CA, Fadok VA, et al. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: Calreticulin and CD91 as a common collectin receptor complex. J Immunol 2002; 169: 3978-3986.
Pickering MC, Fischer S, Lewis MR, Walport MJ, Botto M, Cook HT. Ultraviolet-radiation-induced keratinocyte apoptosis in C1q-deficient mice. J Invest Dermatol 2001; 117: 52-58.
Ren Y, Savill J. Proinflammatory cytokines potentiate thrombospondin-mediated phagocytosis of neutrophils undergoing apoptosis. J Immunol 1995; 154: 2366-2374.
Galati G, Rovere P, Citterio G, et al. In vivo administration of GM-CSF promotes the clearance of apoptotic cells: Effects on monocytes and polymorphonuclear leukocytes. J Leukoc Biol 2000; 67: 174-182.
Scheinecker C, McHugh R, Shevach EM, Germain RN. Constitutive presentation of a natural tissue autoantigen exclusively by dendritic cells in the draining lymph node. J Exp Med 2002; 196: 1079-1090.
Huang FP, Platt N, Wykes M, et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med 2000; 191: 435-444.
Ferguson TA, Herndon J, Elzey B, Griffith TS, Schoenberger S, Green DR. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8(+) T cells produce active immune unresponsiveness. J Immunol 2002; 168: 5589-5595.
Liu K, Iyoda T, Saternus M, Kimura Y, Inaba K, Steinman RM. Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med 2002; 196: 1091-1097.
Valdez Y, Mah W, Winslow MM, Xu L, Ling P, Townsend SE. Major histocompatibility complex class II presentation of cell-associated antigen is mediated by CD8alpha+ dendritic cells in vivo. J Exp Med 2002; 195: 683-694.
Belz GT, Behrens GM, Smith CM, et al. The CD8alpha(+) dendritic cell is responsible for inducing peripheral selftolerance to tissue-associated antigens. J Exp Med 2002; 196: 1099-1104.
Mougneau E, Hugues S, Glaichenhaus N. Antigen presentation by dendritic cells in vivo. J Exp Med 2002; 196: 1013- 1016.
O'Brien BA, Huang Y, Geng X, Dutz JP, Finegood DT. Phagocytosis of apoptotic cells by macrophages from NOD mice is reduced. Diabetes 2002; 51: 2481-2488.
Zhang Y, O'Brien B, Trudeau J, Tan R, Santamaria P, Dutz JP. In Situ beta cell death promotes priming of diabetogenic CD8 T lymphocytes. J Immunol 2002; 168: 1466-1472.
Hugues S, Mougneau E, Ferlin W, et al. Tolerance to islet antigens and prevention from diabetes induced by limited apoptosis of pancreatic beta cells. Immunity 2002; 16: 169- 181.
Baumann I., Kolowos W., Voll RE, et al. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis & Rheumatism 2002; 46: 191-201.
Ronnblom L, Alm GV. The natural interferon-alpha producing cells in systemic lupus erythematosus. Hum Immunol 2002; 63: 1181-1193.
Palucka AK, Banchereau J, Blanco P, Pascual V. The interplay of dendritic cell subsets in systemic lupus erythematosus. Immunol Cell Biol 2002; 80: 484-488.
Hardin JA. Directing autoimmunity to nucleoprotein particles: The impact of dendritic cells and interferon alpha in lupus. J Exp Med 2003; 197: 681-685.
Paczesny S, Beranger S, Salzmann JL, Klatzmann D, Colombo BM. Protection of mice against leukemia after vaccination with bone marrow-derived dendritic cells loaded with apoptotic leukemia cells. Cancer Res 2001; 61: 2386- 2389.
Motta I, Andre F, Lim A, et al. Cross-presentation by dendritic cells of tumor antigen expressed in apoptotic recombinant canarypox virus-infected dendritic cells. J Immunol 2001; 167: 1795-1802.
Chen Z, Moyana T, Saxena A, Warrington R, Jia Z, Xiang J. Efficient antitumor immunity derived from maturation of dendritic cells that had phagocytosed apoptotic/necrotic tumor cells. Int J Cancer 2001; 93: 539-548.
Fujita N, Kagamu H, Yoshizawa H, et al. CD40 ligand promotes priming of fully potent antitumor CD4(+) T cells in draining lymph nodes in the presence of apoptotic tumor cells. J Immunol 2001; 167: 5678-5688.
Candido KA, Shimizu K, McLaughlin JC, et al. Local administration of dendritic cells inhibits established breast tumor growth: Implications for apoptosis-inducing agents. Cancer Res 2001; 61: 228-236.
Feng H, Zeng Y, Whitesell L, Katsanis E. Stressed apoptotic tumor cells express heat shock proteins and elicit tumorspecific immunity. Blood 2001; 97: 3505-3512.
Son YI, Mailliard RB, Watkins6SC, Lotze MT. Dendritic cells pulsed with apoptotic squamous cell carcinoma have antitumor effects when combined with interleukin-2. Laryngoscope 2001; 111: 1472-1478.
Kotera Y, Shimizu K, Mule JJ. Comparative analysis of necrotic and apoptotic tumor cells as a source of antigen(s) in dendritic cell-based immunization. Cancer Res 2001; 61: 8105-8109.
Nikitina EY, Gabrilovich DI. Combination of gammairradiation and dendritic cell administration induces a potent antitumor response in tumor-bearing mice: Approach to treatment of advanced stage cancer. Int J Cancer 2001; 94: 825-833.
Masse D, Voisine C, Henry F, et al. Increased Vaccination Efficiency with Apoptotic Cells by Silica-induced, Dendriticlike Cells. Cancer Res 2002; 62: 1050-1056.
Castiglioni P, Martin-Fontecha A, Milan G, et al. Apoptosisdependent Subversion of the T-Lymphocyte Epitope Hierarchy in Lymphoma Cells. Cancer Res 2002; 62: 1116- 1122.
Scheffer SR, Nave H, Korangy F, et al. Apoptotic, but not necrotic, tumor cell vaccines induce a potent immune response in vivo. Int J Cancer 2003; 103: 205-211.
Feng H, Zeng Y, Graner MW, Likhacheva A, Katsanis E. Exogenous stress proteins enhance the immunogenicity of apoptotic tumor cells and stimulate antitumor immunity. Blood 2003; 101: 245-252.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rovere-Querini, P., Dumitriu, I.E. Corpse disposal after apoptosis. Apoptosis 8, 469–479 (2003). https://doi.org/10.1023/A:1025538324077
Issue Date:
DOI: https://doi.org/10.1023/A:1025538324077