Abstract
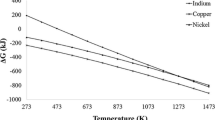
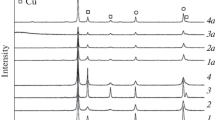
Cu–Ni alloy powders were synthesized directly from metal nitrate solution. Combustion, ultrasonic mist combustion and ultrasonic pyrolysis processes were applied and Cu–Ni alloy powder was successfully synthesized by mist combustion and ultrasonic pyrolysis of nitrate salts in a reducing atmosphere. X-ray diffraction data showed that the copper and the nickel atoms were completely mixed. For Cu–Ni alloy powder prepared by ultrasonic mist combustion, powder was a hollow sphere and consisted of nano-sized particles. For Cu–Ni alloy powder prepared by ultrasonic pyrolysis, particles consisted of nano-scale particles loosely coagulated. The synthesis temperature was 800°C, which is much lower than the liquidus of a Cu–Ni binary system. Metal alloying by mist pyrolysis, named chemical alloying, has many advantages: (1) no crucible, ball or jar is needed, (2) low synthesis temperature below the liquidus of the alloy system, (3) no extraction step is needed, (4) no cation contamination, (5) direct synthesis of fine powder from metal salts and (6) a simple and inexpensive process. The disadvantage is the contamination of organic elements from the solvent and the salt.
Similar content being viewed by others
References
Bhaduri S.B., R. Radhakrishnan & D. Linch, 1994. Ceram. Eng. Sci. Proc. 15, 694.
Chick L.A., J.L. Bates, L.R. Pederson & H.E. Kinginger, 1989. In: Singhal S.C. ed. In Proc. 1st Int'l Symp. Solid Oxide Fuel Cells. Electrochemical Society, Pennington, NJ, p. 170.
Eroglu S., S.C. Zhang & G.L. Messing, 1996. J. Mater. Res. 11, 2131
Jung C.H., 1999. J. Mater. Sci. Lett. 18, 67–70.
Jung C.H. & D.K. Kim, 2002. J. Mater. Synth. Process. 10(1), 23.
Jung C.H., H.G. Lee & G.W. Hong, 2001. J. Mater. Synth. Process. 9(1), 19.
Kim S.J. & C.H. Jung, 1996. J. Mater. Synth. Process. 4(6), 405.
Kanno Y. & T. Suzuki, 1988. J. Mater. Sci. 23, 3067.
Metals reference book, 1976. C.J. Smithells, ed. 5th edn, Butterworths, London & Boston, pp. 119 and 122 (1) and p. 586 (2).
Pluym T.C., T.T. Kodas, L.M. Wang & H.D. Glicksman, 1995. J. Mater. Res. 10, 1661.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jung, CH., Lee, HG., Kim, CJ. et al. Synthesis of Cu–Ni Alloy Powder Directly from Metal Salts Solution. Journal of Nanoparticle Research 5, 383–388 (2003). https://doi.org/10.1023/A:1025510910814
Issue Date:
DOI: https://doi.org/10.1023/A:1025510910814