Abstract
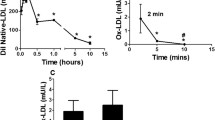
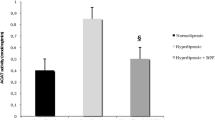
The oxidative modification of low density lipoprotein (LDL) is thought to play an important role in atherogenesis. Drugs of β-hydroxy-β-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) family are usually used as a very effective lipid-lowering preparations but they simultaneously block biosynthesis of both cholesterol and ubiquinone Q10 (coenzyme Q), which is an intermediate electron carrier in the mitochondrial respiratory chain. It is known that reduced form of ubiquinone Q10 acts in the human LDL as very effective natural antioxidant. Daily per os administration of HMG-CoA reductase inhibitor simvastatin to rats for 30 day had no effect on high-energy phosphates (adenosin triphosphate, creatine phosphate) content in liver but decreased a level of these substances in myocardium. We study the Cu2+-mediated susceptibility of human LDL to oxidation and the levels of free radical products of LDL lipoperoxidation in LDL particles from patients with atherosclerosis after 3 months treatment with natural antioxidants vitamin E as well as during 6 months administration of HMG-CoA reductase inhibitors such as pravastatin and cerivastatin in monotherapy and in combination with natural antioxidant ubiquinone Q10 or synthetic antioxidant probucol in a double-blind placebo-controlled trials. The 3 months of natural antioxidant vitamin E administration (400 mg daily) to patients did not increase the susceptibility of LDL to oxidation. On the other hand, synthetic antioxidant probucol during long-time period of treatment (3–6 months) in low-dose (250 mg daily) doesn't change the lipid metabolism parameters in the blood of patients but their high antioxidant activity was observed. Really, after oxidation of probucol-contained LDL by C-15 animal lipoxygenase in these particles we identified the electron spin resonance signal of probucol phenoxyl radical that suggests the interaction of LDL-associated probucol with lipid radicals in vivo. We observed that 6 months treatment of patients with pravastatine (40 mg daily) or cerivastatin (0.4 mg daily) was followed by sufficiently accumulation of LDL lipoperoxides in vivo. In contrast, the 6 months therapy with pravastatin in combination with ubiquinone Q10 (60 mg daily) sharply decreased the LDL initial lipoperoxides level whereas during treatment with cerivastatin in combination with probucol (250 mg daily) the LDL lipoperoxides concentration was maintained on an invariable level. Therefore, antioxidants may be very effective in the prevention of atherogenic oxidative modification of LDL during HMG-CoA reductase inhibitors therapy.
Similar content being viewed by others
References
Steinberg D, Witztum JL: Lipoproteins and atherogenesis — current concepts. JAMA 264: 3047-3052, 1990
Davignon J, Laaksonen R: Low-density lipoprotein-independent effects of statins. Curr Opin Lipidol 10: 543-559, 1999
Gibbons GF, Mitropoulos KA, Myant NB: In: Biochemistry of Cholesterol. Biomedical Press, Amsterdam, 1982, pp 109-130
Folkers K, Langsjoen P, Willis R, Richardson P, Xia LJ, Ye CQ, Tamagawa H: Lovastatin decreases coenzyme Q levels in humans. Proc Natl Acad Sci USA 87: 8931-8934, 1990
Laaksonen R, Jokelainen K, Sahi T, Tikkanen MJ, Himberg JJ: Decreases in serum ubiquinone concentrations do not result in reduced levels in muscle tissue during short-term simvastatin treatment in humans. Clin Pharmacol Ther 57: 62-66, 1995
Martinez-Garcia FA, Martin-Fernandez J, Molto JM, Villaverde R, Morales A, Fernandez-Barreiro A: Myopathy caused by inhibitors of hydroxymethylglutaryl-coenzyme A reductase. Rev Neurol 25: 869-871, 1997
Steinberg D: Role of oxidized LDL and antioxidants in atherosclerosis. In: J.B. Longenecker et al. (eds). Nutrition and Biotechnology in Heart Disease and Cancer. Plenum Press, New York, 1995, pp 39-48
Stocker R, Bowry VW, Frei B: Ubiquinol-10 protect human low density lipoprotein more efficiently against lipid peroxidation than does α-tocopherol. Proc Natl Acad Sci USA 88: 1646-1650, 1991
Cadenas E: Mechanisms of oxygen activation and reactive oxygen species detoxification. In: S. Ahmad (ed). Oxidative Stress and Antioxidant Defenses in Biology. Chapman and Hall, New York, 1995, pp 1-61
Arrigoni O, Dipierro S, Borraccino G: Ascorbate free radical reductase, a key enzyme of the ascorbic acid system. FEBS Lett 125: 242-244, 1981
Maellaro E, Del Bello B, Sugherini L, Santucci A, Comporti M, Casini AF: Purification and characterization of glutathione-dependent dehydroascorbate reductase from rat liver. Biochem J 301: 471-476, 1994
Del Bello B, Maellaro E, Sugherini L, Santucci A, Comporti M, Casini AF: Purification of NADPH-dependent dehydroascorbate reductase from rat liver and its identification with 3α-hydroxysteroid dehydrogenase. Biochem J 304: 385-390, 1994
Mortensen S, Leth A, Agner E, Rohde M: Dose-related decrease of serum coenzyme Q-10 during treatment with HMG-CoA reductase inhibitors. Mol Aspects Med 18: S137-S144, 1997
Palomaki A, Malminiemi K, Sovakivi T, Malminiemi O: Ubiquinone supplementation during lovastatin treatment: Effect on LDL oxidation ex vivo. J Lipid Res 39: 1430-1437, 1998
Lamprecht W, Stein P, Henz F, Weisser H: Creatine phosphate determination with creatine kinase, hexokinase, and glucose-6-phosphate dehydrogenase. In: H.U. Bergmeyer (ed). Methods of Enzymatic Analysis, vol. 4. Academic Press, New York, 1974, pp 1777-1781
Pisarenko OI, Tskitishvily OV, Studneva IM, Serebryakova LI, Korchazhkina OV: Metabolic effects of ischemic preconditioning and adenosine receptor blockade in dogs. Ann NY Acad Sci 793: 85-97, 1996
Reimer KA, Hill ML, Jennings RB: Prolonged depletion of ATP and of adenine nucleotide pool due to delayed resynthesis of adenine nucleotides following reversible myocardial ischemic injury in dogs. J Mol Cell Cardiol 13: 229-239, 1981
Tertov VV, Kaplun VV, Dvoryantsev SN, Orekhov AN: Apolipoprotein B-bound lipids as a marker for evaluation of low density lipoprotein oxidation in vivo. Biochem Biophys Res Commun 214: 608-613, 1995
Lindgren RF: Preparative ultracentrifugal laboratory procedure suggestions for lipoprotein analysis. In: E.G. Perkins (ed). Analysis of Lipids and Lipoproteins. American Oil Chemical Society, New York, 1975, pp 204-224
Tikhaze AK, Lankin VZ, Kolycheva SN, Konovalova GG, Shumaev KB, Kozachenko AI, Gurevich SM, Zharova EA, Smirnov LD: Does trimetazidine act as antioxidant? Bull Exp Biol Med 126: 1132-1134, 1998
Lankin VZ, Gordeeva NT, Osis YuG, Vikhert AM, Schewe T, Rapoport SM: Animal lipoxygenases: Change in activity of lipoxygenase from reticulocytes upon interaction with blood plasma lipoproteins. Biochemistry (Moscow) 48: 782-788, 1983
Lankin VZ, Kuhn H, Hiebsch C, Schewe T, Rapoport S, Tikhaze AK, Gordeeva NT: On the nature of the stimulation of the lipoxygenase from rabbit reticulocytes by biological membranes. Biomed Biochim Acta 44: 655-664, 1985
Shumaev KB, Ruuge EK, Dmitrovsky AA, Bykhovsky VYa, Kukharchuk VV: Effect of lipid peroxidation products and antioxidants on the formation of probucol radical in low density lipoproteins. Biochemistry (Moscow) 62: 657-660, 1997.
Nourooz-Zadeh J, Tajaddini-Sarmadi J, Wolf SP: Measurement of plasma hydroperoxide concentrations by the ferrous oxidation — xylenol orange assay in conjunction with triphenylphosphine. Analyt Biochem 220: 403-409, 1994
Tikhaze AK, Lankin VZ, Konovalova GG, Shumaev KB, Kaminnyi AI, Kozachenko AI, Gurevich SM, Nagler LG, Zaitseva TM, Kukharchuk VV: Antioxidant probucol as an effective scavenger of lipid radicals in low density lipoproteins in vivo and in vitro. Bull Exp Biol Med 128: 818-821, 1999
Kuzuya M, Kuzuya F: Probucol as an antioxidant and antiatherogenic drug. Free Radic Biol Med 14: 67-77, 1993
Cristol LS, Jialal I, Grundy SM: Effect of low-dose probucol therapy on LDL oxidation and the plasma lipoprotein profile in male volunteers. Atherosclerosis 97: 11-20, 1992
Dujovne CA, Harris WS, Gerrond LLC: Comparison of effects of probucol vs. vitamin E on ex vivo oxidation susceptibility of lipoproteins in hyperlipoproteinemia. Am J Cardiol 74: 38-42, 1994
Rodes J, Cote G, Lesperance J, Bourassa M, Doucet S, Bilodeau L, Bertrand OF, Hagel F, Gallo R, Tardif JC: Prevention of restenosis after angioplasty in small coronary arteries with probucol. Circulation 97: 429-436, 1998
Dujovne CA: New lowering drugs and new effects of old drugs. Curr Opin Lipidol 8: 362-368, 1997
Lankin VZ, Tikhaze AK, Kotelevtseva NV: Lipid peroxides and atherosclerosis. Kardiologiia (Cardiology) 16: 23-30, 1976 [Article in Russian; abstract in English]
Lankin VZ: Lipid peroxides and atherosclerosis. Hypothesis: The role of cholesterol and free radical lipid peroxidation in altering cell membrane properties during hypercholesterolemia and atherosclerosis. Kardiologiia (Cardiology) 20: 42-48, 1980 [Article in Russian; abstract in English]
Lankin VZ: Atherosclerosis as a free radical pathology. Excerpta Med (Int Congr Ser) G98: 385-388, 1992
Lankin VZ: Free radical lipoperoxidation during atherosclerosis. Free Radic Biol Med 16: 8, 1994
Belkner J, Wiesner R, Kuhn H, Lankin VZ: The oxygenation of cholesterol esters by the reticulocyte lipoxygenase. FEBS Lett 279: 110-114, 1991
Kuhn H, Belkner J, Wiesner R, Schewe T, Lankin VZ, Tikhaze AK: Structure elucidation of oxygenated lipids in human atherosclerotic lesions. Eicosanoids 5: 17-22, 1992
Lankin VZ, Vikhert AM, Kosykh VA, Tikhaze AK, Galakhov IE, Orekhov AN, Repin VN: Enzymatic detoxication of superoxide anion-radical and lipoperoxides in intima and media of atherosclerotic aorta. Biomed Biochim Acta 43: 797-802, 1984
Osis YuG, Formaziuk VE, Lankin VZ, Dudina EI, Vikhert AM, Vladimirov YuA: The chemiluminescence of different classes lipoproteins from human blood serum. Vopr Med Khim (Problems of Medical Chemistry) 28: 122-126, 1982 [Article in Russian; abstract in English]
Wills RA, Folkers K, Lan Tucker J, Chun-Qu Y, Li-Jun X, Tamagawa H: Lovastatin decreases coenzyme Q levels in rats. Proc Natl Acad Sci USA 87: 8928-8930, 1990
Tikhaze AK, Lankin VZ, Mikhin VP, Revenko VM, Lupanov VP: The antioxidant probucol as a regulator of the intensity of free radical lipid peroxidation processes in the blood of patients with coronary atherosclerosis. Ter. Arkh. (Therapeutic Archive) 69: 35-41, 1997 [Article in Russian; abstract in English]
Kumar D, Palace V, Danelisen I, Jugdutt BI, Singal PK: Probucol induced antioxidant confers protection against ischemia-reperfusion injury. J Mol Cell Cardiol. 33: A62, 2001
Lankin VZ, Tikhaze AK,.Konovalova GG, Kukharchuk VV: HMG-CoA reductase inhibitors induced the LDL oxidation. J Mol Cell Cardiol 33: A65, 2001
Mannuel y Kennoy B, Vertommen J, Vinckx M, De Leeuw L: Effects of atorvastatin and and vitamin E on lipid peroxidation in patients with type I diabetes melitus. In: Abstr. Book Int. Conf. 'Free Radicals, Nitric Oxide, and Inflammation: Molecular, Biochemical, and Clinical Aspects'. NATO-ASI, Anthalya (Turkey), 2001, p 83
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lankin, V.Z., Tikhaze, A.K., Kukharchuk, V.V. et al. Antioxidants decreases the intensification of low density lipoprotein in vivo peroxidation during therapy with statins. Mol Cell Biochem 249, 129–140 (2003). https://doi.org/10.1023/A:1024742907379
Issue Date:
DOI: https://doi.org/10.1023/A:1024742907379