Abstract
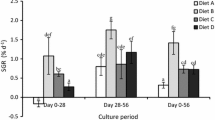
Carp larvae, like any other fish larvae dependon natural food during first few days of theirlife. In nursery conditions, high mortality andslow larval growth are of common occurrence;sub-optimal nutrition might be a possiblereason for such consequences. To improve thesituation the effect of feeding ascorbicacid-enriched live food on survival, growth,tissue biochemical composition includingascorbate level was evaluated in first feeding(3 days old) larvae (av. wt. 2.2 mg) of therohu carp, Labeo rohita (Ham.) for aperiod of 15 days (temp. 28.6 ± 1 °C)under natural photoperiod. The larvae (stockingdensity 10 l−1) were offered enriched andnon-enriched zooplankton ad libitumfollowing a rigid schedule with four feedingregimes, each having 3 replicates. In treatmentT1, non-enriched zooplankton (Moina,Daphnia, Cyclops, Diaptomus) and in T2,T3, T4 ascorbic acid enriched (12 henrichment) zooplankton [@10%, 20% and 30%ascorbyl palmitate (AP) inclusion in diet ofzooplankton] were offered. Highest survival(90%) and growth (9563% live weight gain)could be seen in T3 group and the lowestin T1 (62% survival and 805% live weightgain), thus confirming the dietary essentialityof ascorbic acid for rohu larvae. Therequirement has been shown to be 1409 µg/gdry diet. Whole body tissue analyses for crudeprotein, total lipid and RNA: DNA ratiofollowed the same trend as that of growthresponse and percent survival. Significantpositive correlation (r = 0.949 and 0.861) couldbe found with muscle RNA/DNA ratio and muscleRNA content with specific growth rate indifferent treatments. Significant differencewas found in tissue ascorbate levels betweenenriched plankton fed groups, being highest in T3. Such live foodmediated vitamin transfer might be an effectivemeans to provide higher plane of nutrition forhigh survival and rapid growth for rohu larva.
Similar content being viewed by others
References
AOAC 1998. Official Methods of Analysis, 16th edition. Association of Official Analytical Chemists, AOAC International, Gaithersburg, Maryland, 20877-2417, Vol. 1, Chapter 4, pp. 1–43.
APHA 1989. Standard Methods for the. Examination of Water and Wastewater. American Public Health Association, 17th edition. Washington, DC, Part 4000, pp. 1–208.
Bastrop R., Jurss K. and Wacke R. 1992. Biochemical parameters as a measure of food availability and growth in immature rainbow trout (Oncorhynchus mykiss). Comparative Biochemistry and Physiology 102A: 151–161.
Bulow J.F., Coburn C.B. and Cobb C.S. 1978. Comparison of two bluegill populations by means of the RNA-DNA ratios and liver-somatic index. Transactions of American Fisheries Society 107: 799–803.
Charlon N. and Bergot P. 1984. Rearing system for feeding fish larva on dry diets: Trial with carp (Cyprinus carpio L.) larvae. Aquaculture 41: 1–9.
Chaterjee G.C. 1967. Effects of ascorbic acid deficiency in animals. In: W.H. Sebrell, Jr. and R.S. Harris (eds), The Vitamins. Vol. 1. Academic Press, New York and London, pp. 407–456.
Chen H.Y. and Chang C.F. 1994. Quantification of vitamin C requirements for juvenile shrimp (Penaeus monodon) using polyphosphorylated l-ascorbic acid. Journal of Nutrition 124: 2033–2038.
Clemmesen C.M. 1987. Laboratory studies on RNA/DNA ratios of starved and fed herring (Clupea harengus) and turbot (Scophthalmus maximus) larvae. Journal du Conseil International de l'Exploration du Mer 43: 122–128
Dabrowski K. 1990. Ascorbic acid status in the early life of whitefish (Coregonus lavaretus). Aquaculture 84: 61–70
Dabrowski K. 1992. Ascorbate concentration in fish ontogeny. Journal of Fish Biology 40: 273–279.
Foster A.R., Houlihan D.F., Hall S.J. and Burren L.J. 1992. The effect of temperature acclimation on protein synthesis rates and nucleic acid content of juvenile cod (Gadus morhua L.). Canadian Journal of Zoology 70: 2095–2102.
Fu C., Cui Y., Hung S.S.O. and Zhu Z. 1998. Growth and feed utilizaion by F4 human growth hormone transgenic carp fed diets with different protein levels. Journal of Fish Biology 53: 115–129.
Gouillou-Coustans M.F., Bergot P. and Kaushik S.J. 1998. Dietary ascorbic acid needs of common carp (Cyprinus carpio) larvae. Aquaculture 161: 453–461.
Jagota S.K. and Dani H.M. 1982. A new colorimetric technique for the estimation of vitamin C using folin phenol reagent. Clinical Biochemistry 127: 178–182.
Jaffe G.M. 1984. Vitamin C. In: L.J. Machlin (ed), Handbook of Vitamins. Marcel Dekker, New York, NY, pp. 199–244.
Jena J.K., Aravindakshan P.K. and Sing W.J. 1998. Nursery rearing of Indian major carp fry under different stocking densities. Indian Journal of Fisheries 45(2): 163–168.
Kaushik S.J. 1985. Some aspects of larval nutritional physiology in carp. In: R. Billard and J. Marcel (eds), Aquaculture des Cyprinides. INRA Publ, pp. 215–226.
Kaushik S.J., Gouillou-Coustans M.F. and Cho C.Y. 1998. Application of the recommendations on vitamin requirements of finfish by NRC (1993) to salmonids and sea bass using practical and purified diets. Aquacualture 161: 463–474.
Mahajan C. and Agrawal N.K. 1980. Nutritional requirement of ascorbic acid by Indian major carp, Cirrhina mrigala, during early growth. Aquaculture 19: 37–48.
Merchie G., Lavens P., Nelis H, De Leenheer L. and Sorgeloss P. 1993. Effect of vitamin C incorporation in live food on the larviculture success of aquaculture species. In: Medical Faculty of Landbouw. University Ghent 58/4b.
Merchie G., Lavens P. and Sorgeloos P. 1997a. Optimization of dietary vitamin C in fish and crustacean larvae: A review. Aquaculture 55: 165–181.
Merchie G., Lavens P., Verreth J., Ollevier F., Nelis H., Deleenheer A., Storch V. and Sorgeloos P. 1997b. The effect of supplemental ascorbic acid in enriched live food for Clarias gariepinus larvae at start feeding. Aquaculture 151: 245–258.
Miglavs I. and Jobling M. 1989. Effects of feeding regime on food consumption, growth rates and tissue nucleic acids in juvenile arctic charr, Salvelinus alpinus, with particular respect to compensatory growth. Journal of Fish Biology 34: 947–957.
Mishra S. and Mukhopadhyay P.K. 1996. Ascorbic acid requirement of catfish by Clarias batrachus (Linn.) Indian Journal of Fisheries 2: 157–187.
Mitra Gopa and Mukhopadhyay P.K. 2002. Growth, nutrient utilisation and tissue biochemical changes in Labeo rohita, fed with natural and prepared diet. Journal of Applied Aquaculture 12(3): 65–80.
Mookherji N. and Rao T.R. 1991. Survival and growth of Rohu (Labeo rohita) and Singhi (Heteropneustes fossilis) larvae fed on dry and live foods. In: P. Lavens, P. Sorgeloos, E. Jaspers and F. Ollevier (eds), European Aquaculture Society. Special Publication, Vol. 15, Larvi 1991, Fish and Crustacean larviculture symposium, Belgium, pp. 148–150.
Moreau R. and Dabrowski K. 2001. Gulonolactone oxidase presence in fishes: activity and significance. In: K. Dabrowski (ed), Ascorbic Acid in Aquatic Organisms, Status and Perspectives. CRC Press, Boca Raton, London, New York, Washington, D.C., pp. 14–32.
Mukhopadhyay P.K., Nandi S., Hassan M.A., Dey A. and Sarkar S. 1998. Effect of dietary deficiency and supplementation of ascorbic acid on performance, vertebral collagen content, tissue vitamin and enzyme status in Labeo rohita, In: M.S Hameed and B.M. Kurup (eds), Technological Advancement in Fisheries, Publ. 1, Cochin University of Science and Technology, Cochin, pp. 101–107.
Sorgeloos P. and Leger P.L. 1992. Improved larviculture outputs of marine fish, shrimp and prawn. Journal of World Aquaculture Society 23: 251–264.
Shiau S.Y. and Jans F.L. 1992. Dietary ascorbic acid requirement of juvenile tilapia Oreochromis niloticus × O. aureus. Nippon Suisan Gakkaishi 58(4): 671–675.
Woodward B. 1994. Dietary vitamin requirements of cultured young fish, with emphasis on quantitative estimates for salmonids. Aquaculture 124: 133–168.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mitra, G., Mukhopadhyay, P. Dietary essentiality of ascorbic acid in rohu larvae: Quantification with ascorbic acid enriched zooplankton. Aquaculture International 11, 81–93 (2003). https://doi.org/10.1023/A:1024158226110
Issue Date:
DOI: https://doi.org/10.1023/A:1024158226110