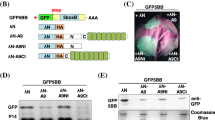
Abstract
The Arabidopsis genome contains seven genes that belong to the RecQ family of ATP- dependent DNA helicases. RecQ members in Saccharomyces cerevisiae (SGS1) and man (WRN, BLM and RecQL4) are involved in DNA recombination, repair and genome stability maintenance, but little is known about the function of their plant counterparts. The Arabidopsis thaliana RecQsim gene is remarkably different from the other RecQ-like genes due to an insertion in its helicase domain. We isolated the AtRecQsim orthologues from rice and rape and established the presence of a similar insertion in their helicase domain, which suggests a plant specific function for the insert. The expression pattern of the AtRecQsim gene was compared with the other Arabidopsis RecQ-like members in different tissues and in response to stress. The transcripts of the AtRecQsim gene were found in all plant organs and its accumulation was higher in roots and seedlings, as compared to the other AtRecQ-like members. In contrast to most AtRecQ-like genes, the examined environmental cues did not have a detectable effect on the accumulation of the AtRecQsim transcripts. The budding yeast sgs1 mutant, which is known to be hypersensitive to the DNA-damaging drug MMS, was transformed with the AtRecQsim cDNA. The AtRecQsim gene suppressed the MMS hypersensitivity phenotype of the sgs1 cells. We propose that the Arabidopsis RecQsim gene, despite its unusual structure, exhibits an evolutionary conserved function.
Similar content being viewed by others
References
Arabidopsis Genome Initiative. 2000. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815.
Aubourg, S., Kreis, M. and Lecharny, A. 1999. The DEAD box RNA helicase family in Arabidopsis thaliana. Nucl. Acids Res. 27: 628–636.
Bertolaet, B.L., Clarke, D.J., Wolff, M., Watson, M.H., Henze, M., Divita, G. and Reed, S.I. 2001. UBA domains mediate protein-protein interactions between two DNA damage-inducible proteins. J. Mol. Biol. 313: 955–963.
Boudet, N., Aubourg, S., Toffano-Nioche, C., Kreis, M. and Lecharny, A. 2001. Evolution of intron/exon structure of DEAD helicase family genes in Arabidopsis, Caenorhabditis, and Drosophila. Genome Res. 11: 2101–2114.
Chakraverty, R.K. and Hickson, I.D. 1999. Defending genome integrity during DNA replication: a proposed role for RecQ family helicases. Bioessays 21: 286–294.
Ellis, N.A., Groden, J., Ye, T.Z., Straughen, J., Lennon, D.J., Ciocci, S., Proytcheva, M. and German, J. 1995. The Bloom's syndrome gene product is homologous to RecQ helicases. Cell 83: 655–666.
Frei, C. and Gasser, S.M. 2000. The yeast Sgs1p helicase acts upstream of Rad53p in the DNA replication checkpoint and colocalizes with Rad53p in S-phase-specific foci. Genes Dev. 14: 81–96.
Fricke, W.M., Kaliraman, V. and Brill, S.J. 2001. Mapping the DNA topoisomerase III binding domain of the Sgs1 DNA helicase. J. Biol. Chem. 276: 8848–8855.
Fukuchi, K., Martin, G.M. and Monnat, R.J. Jr. 1989. Mutator phenotype of Werner syndrome is characterized by extensive deletions. Proc. Natl. Acad. Sci. USA 86: 5893–5897.
Gangloff, S., McDonald, J.P., Bendixen, C., Arthur, L. and Rothstein, R. 1994. The yeast type I topoisomerase Top3 interacts with Sgs1, a DNA helicase homolog: a potential eukaryotic reverse gyrase. Mol. Cell Biol. 14: 8391–8398.
Ge, Q., Frank, M.B., O'Brien, C. and Targoff, I.N. 1992. Cloning of a complementary DNA coding for the 100 KD antigenic protein of the PM-Scl autoantigen. J. Clin. Invest. 90: 559–570.
Gee, J., Ding, Q. and Keller, J.N. 2002. Analysis ofWerner's expression within the brain and primary neuronal culture. Brain Res. 14: 44–48.
Gorbalenya, A.E., Koonin, E.V., Donchenko, A.P. and Blinov, V.M. 1989. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucl. Acids Res. 17: 4713–4730.
Haeseleer, F., Imanishi, Y., Sokal, I., Filipek, S. and Palczewski, K. 2002. Calcium-binding proteins: intracellular sensors from the calmodulin superfamily. Biochem. Biophys. Res. Commun. 290: 615–623.
Hanada, K., Ukita, T., Kohno, Y., Saito, K., Kato, J. and Ikeda, H. 1997. RecQ DNA helicase is a suppressor of illegitimate recombination in Escherichia coli. Proc. Natl. Acad. Sci. USA 94: 3860–3865.
Hartung, F., Plchova, H. and Puchta, H. 2000. Molecular characterization of RecQ homologues in Arabidopsis thaliana. Nucl. Acids Res. 28: 4275–4282.
Heo, S.J., Tatebayashi, K., Ohsugi, I., Shimamoto, A., Furuichi, Y. and Ikeda, H. 1999. Bloom's syndrome gene suppresses premature ageing caused by Sgs1 deficiency in yeast. Genes Cells 4: 619–625.
Hu, P., Beresten, S.F., van Brabant, A.J., Ye, T.Z., Pandolfi, P.P., Johnson, F.B., Guarente, L. and Ellis, N.A. 2001. Evidence for BLM and Topoisomerase IIIα interaction in genomic stability. Hum. Mol. Genet. 10: 1287–1298.
Imamura, O., Fujita, K., Shimamoto, A., Tanabe, H., Takeda, S., Furuichi, Y. and Matsumoto, T. 2001. Bloom helicase is involved in DNA surveillance in early S phase in vertebrate cells. Oncogene 20: 1143–1151.
Isono, K., Yamamoto, H., Satoh, K. and Kobayashi, H. 1999. An Arabidopsis cDNA encoding a DNA-binding protein that is highly similar to the DEAH family of RNA/DNA helicase genes. Nucl. Acids Res. 27: 3728–3735.
Jacobsen, S.E., Running, M.P. and Meyerowitz, E.M. 1999. Disruption of an RNA helicase/RNAse III gene in Arabidopsis causes unregulated cell division in floral meristems. Development 126: 5231–5243.
Kanaya, E., Nakajima, N. and Okada, K. 2002. Non-sequencespecific DNA binding by the filamentous flower protein from Arabidopsis thaliana is reduced by EDTA. J. Biol. Chem. 277: 11957–11964.
Karow, J.K., Chakraverty, R.K. and Hickson, I.D. 1997. The Bloom's syndrome gene product is a 3′-5′ DNA helicase. J. Biol. Chem. 272: 30611–30614.
Kawabe, T., Tsuyama, N., Kitao, S., Nishikawa, K., Shimamoto, A., Shiratori, M., Matsumoto, T., Anno, K., Sato, T., Mitsui, Y., Seki, M., Enomoto, T., Goto, M., Ellis, N.A., Ide, T., Furuichi, Y. and Sugimoto, M. 2002. Differential regulation of human RecQ family helicases in cell transformation and cell cycle. Oncogene 28: 4764–4772.
Kitao, S., Lindor, N.M., Shiratori, M., Furuichi, Y. and Shimamoto, A. 1999. Rothmund-Thomson syndrome responsible gene, RECQL4: genomic structure and products. Genomics 61: 268–276.
Kusano, K., Berres, M.E. and Engels, W.R. 1999. Evolution of the RECQ family of helicases: a Drosophila homolog, Dmblm, is similar to the human Bloom syndrome gene. Genetics 151: 1027–1039.
Lebel, E.G., Masson, J., Bogucki, A. and Paszkowski, J. 1993. Stress-induced intrachromosomal recombination in plant somatic cells. Proc. Natl. Acad. Sci. USA 90: 422–426.
Lindor, N.M., Furuichi, Y., Kitao, S., Shimamoto, A., Arndt, C. and Jalal, S. 2000. Rothmund-Thomson syndrome due to RECQ4 helicase mutations: report and clinical and molecular comparisons with Bloom syndrome and Werner syndrome. Am. J. Med. Genet. 90: 223–228.
Liu, Z., Macias, M.J., Bottomley, M.J., Stier, G., Linge, J.P., Nilges, M., Bork, P. and Sattler, M. 1999. The three-dimensional structure of the HRDC domain and implications for the Werner and Bloom syndrome proteins. Structure Fold. Des. 7: 1557–1566.
Lu, J., Mullen, J.R., Brill, S.J., Kleff, S., Romeo, A.M. and Sternglanz, R. 1996. Human homologues of yeast helicase. Nature 383: 678–679.
Lucht, J.M., Mauch-Mani, B., Steiner, H.Y., Metraux, J.P., Ryals, J. and Hohn, B. 2002. Pathogen stress increases somatic recombination frequency in Arabidopsis. Nature Genet. 303: 311–314.
Mankouri, H.W., Craig, T.J. and Morgan, A. 2002. SGS1 is a multicopy suppressor of srs2: functional overlap between DNA helicases. Nucl. Acids Res. 30: 1103–1113.
Matson, S.W., Bean, D.W. and George, J.W. 1994. DNA helicases: enzymes with essential roles in all aspects of DNA metabolism. Bioessays 16: 13–22.
Michiels, J., Xi, C., Verhaert, J. and Vanderleyden, J. 2002. The functions of Ca(2+) in bacteria: a role for EF-hand proteins? Trends Microbiol. 10: 87–93.
Miyajima, A., Seki, M., Onoda, F., Shiratori, M., Odagiri, N., Ohta, K., Kikuchi, Y., Ohno, Y. and Enomoto, T. 2000a. Sgs1 helicase activity is required for mitotic but apparently not for meiotic functions. Mol. Cell Biol. 20: 6399–6409.
Miyajima, A., Seki, M., Onoda, F., Ui, A., Satoh, Y., Ohno, Y. and Enomoto, T. 2000b. Different domains of Sgs1 are required for mitotic and meiotic functions. Genes Genet. Syst. 75: 319–326.
Moser, M.J., Oshima, J. and Monnat, R.J. Jr. 1999. WRN mutations in Werner syndrome. Hum. Mutat. 13: 271–279.
Mullen, J.R., Kaliraman, V. and Brill, S.J. 2000. Bipartite structure of the SGS1 DNA helicase in Saccharomyces cerevisiae. Genetics 154: 1101–1114.
Mullen, J.R., Kaliraman, V., Ibrahim, S.S. and Brill, S.J. 2001. Requirement for three novel protein complexes in the absence of the Sgs1 DNA helicase in Saccharomyces cerevisiae. Genetics 157: 103–118.
Nakayama, K., Irino, N. and Nakayama, H. 1985. The recQ gene of Escherichia coli K12: molecular cloning and isolation of insertion mutants. Mol. Gen. Genet. 200: 266–271.
Neff, N.F., Ellis, N.A., Ye, T.Z., Noonan, J., Huang, K., Sanz, M. and Proytcheva, M. 1999. The DNA helicase activity of BLM is necessary for the correction of the genomic instability of Bloom syndrome cells. Mol. Biol. Cell 10: 665–676.
Ng, S.W., Liu, Y., Hasselblatt, K.T., Mok, S.C. and Berkowitz, R.S. 1999. A new human topoisomerase III that interacts with SGS1 protein. Nucl. Acids Res. 27: 993–1000.
Onoda, F., Seki, M., Miyajima, A. and Enomoto, T. 2000. Elevation of sister chromatid exchange in Saccharomyces cerevisiae sgs1 disruptants and the relevance of the disruptants as a system to evaluate mutations in Bloom's syndrome gene. Mutat. Res. 459: 203–209.
Ouwerkerk, P.B.F., de Kam, R.J., Hoge, J.H.C. and Meijer, A.H. 2001. Glucocorticoid-inducible gene expression in rice. Planta 213: 370–378.
Ries, G., Heller, W., Puchta, H., Sandermann, H., Seidlitz, H.K. and Hohn, B. 2000. Elevated UV-B radiation reduces genome stability in plants. Nature 406: 98–101.
Saffi, J., Pereira, V.R. and Henriques, J.A. 2000. Importance of the Sgs1 helicase activity in DNA repair of Saccharomyces cerevisiae. Curr. Genet. 37: 75–78.
Sambrook, J. and Russell, D.W. 2001. Molecular Cloning: A Laboratory Manual, 3rd ed. Cold Spring Harbor Laboratory Press, Plainview, NY.
Sinclair, D.A. and Guarente, L. 1997. Extrachromosomal rDNA circles: a cause of aging in yeast. Cell 91: 1033–1042.
Sinclair, D.A., Mills, K. and Guarente, L. 1997. Accelerated aging and nucleolar fragmentation in yeast sgs1 mutants. Science 277: 1313–1316.
Sun, H., Bennett, R.J. and Maizels, N. 1999. The Saccharomyces cerevisiae Sgs1 helicase efficiently unwinds G-G paired DNAs. Nucl. Acids Res. 27: 1978–1984.
Suzuki, N., Shimamoto, A., Imamura, O., Kuromitsu, J., Kitao, S., Goto, M., Furuichi, Y. 1997. DNA helicase activity in Werner's syndrome gene product synthesized in a baculovirus system. Nucl. Acids Res. 25: 2973–2978.
Turley, H., Wu, L., Canamero, M., Gatter, K.C. and Hickson, I.D. 2001. The distribution and expression of the Bloom's syndrome gene product in normal and neoplastic human cells. Br. J. Cancer 20: 261–265.
Ui, A., Satoh, Y., Onoda, F., Miyajima, A., Seki, M. and Enomoto, T. 2001. The N-terminal region of Sgs1, which interacts with Top3, is required for complementation of MMS sensitivity and suppression of hyper-recombination in sgs1 disruptants. Mol. Genet. Genomics 265: 837–850.
Valvekens, D., Van Lijsebettens, M. and Van Montagu, M. 1991. Arabidopsis regeneration and transformation (root explant system). In: K. Lindsey (Ed.). Plant Tissue Culture Manual, Kluwer Academic Publishers, Dordrecht, Netherlands, pp. A8: 1–17.
van Brabant, A.J., Stan, R. and Ellis, N.A. 2000. DNA helicases, genomic instability, and human genetic disease. Annu. Rev. Genomics Hum. Genet. 1: 409–459.
Villani, G. and Tanguy, L.G. 2000. Interactions of DNA helicases with damaged DNA: possible biological consequences. J. Biol. Chem. 275: 33185–33188.
von Kobbe, C., Karmakar, P., Dawut, L., Opresko, P., Zeng, X., Brosh, R.M. Jr., Hickson, I.D. and Bohr, V.A. 2002. Colocalization, physical and functional interaction between Werner and Bloom syndrome proteins. J. Biol. Chem. 277: 22035–22044.
Wang, Y., Duby, G., Purnelle, B. and Boutry, M. 2000. Tobacco VDL gene encodes a plastid DEAD box RNA helicase and is involved in chloroplast differentiation and plant morphogenesis. Plant Cell 12: 2129–2142.
Wu, L., Davies, S.L., North, P.S., Goulaouic, H., Riou, J.F., Turley, H., Gatter, K.C. and Hickson, I.D. 2000. The Bloom's syndrome gene product interacts with topoisomerase III. J. Biol. Chem. 275: 9636–9644.
Yamagata, K., Kato, J., Shimamoto, A., Goto, M., Furuichi, Y. and Ikeda, H. 1998. Bloom's and Werner's syndrome genes suppress hyperrecombination in yeast sgs1 mutant: implication for genomic instability in human diseases. Proc. Natl. Acad. Sci. USA 95: 8733–8738.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bagherieh-Najjar, M.B., de Vries, O.M., Kroon, J.T. et al. Arabidopsis RecQsim, a plant-specific member of the RecQ helicase family, can suppress the MMS hypersensitivity of the yeast sgs1 mutant. Plant Mol Biol 52, 273–284 (2003). https://doi.org/10.1023/A:1023968429220
Issue Date:
DOI: https://doi.org/10.1023/A:1023968429220