Abstract
Purpose: It is well established that human granulosa cells and luteal cells express inhibin/activin subunit protein and secrete immunoreactive inhibin. The gonadotropic control of secretion of different molecular forms of inhibin and activin A by granulose–luteal cells (G-LCs) was investigated using recently developed specific enzyme immunoassays (EIAs).
Methods: Granulosa–luteal cells obtained at IVF egg pickup were cultured in a serum-free medium at 37°C in a water-saturated incubator with 5% CO 2 for up to 5 days. Experiments with varying concentrations of human FSH, hLH, and hCG were carried out.
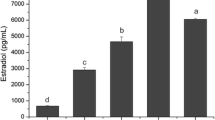
Results: FSH raised the secretion of inhibin A and pro-αC-containing inhibins after 24 and 48 hr in culture. Inhibin B was raised after 24 hr and activin A was raised after 48 hr of FSH treatment. LH treatment for 24 hr stimulated inhibin A, inhibin B, pro-αC, and activin A. hCG stimulated G-LC secretion of inhibin A after 48 hr and pro-αC after 24 hr. Paradoxically, inhibin B secretion was inhibited by 1 and 10 ng/ml hCG after 48 hr. Activin A was stimulated by hCG after 24 and 48 hr of incubation. G-LC secretion of estradiol and progesterone was also stimulated significantly by LH and hCG.
Conclusions: Secretion of dimeric inhibins and activin A is controlled differentially by gonadotropins.
Similar content being viewed by others
REFERENCES
Ling N, Ying S-Y, Ueno N, Shimasaki S, Esch F, Hotta M, Guillemin R: Pituitary FSH is released by a heterodimer of the β subunits from the two forms of inhibin. Nature 1986;321:779–782
Miyamoto K, Hasegawa Y, Fukuda M, Nomura M, Igarashi M, Kangawa K, Matsuo H: Isolation of porcine follicular fluid inhibin of 32 kdaltons. Biochem Biophys Res Commun 1985;129:396–403
Robertson DM, Foulds LM, Leversha L, Morgan FJ, Hearn MTW, Burger HG, Wettenhall REH, de Kretser DM: Isolation of inhibin from bovine follicular fluid. Biochem Biophys Res Commun 1985;126:220–226
Ling N, Ying S-Y, Ueno N, Esch F, Denoroy L, Guillemin R: Isolation and partial characterization of a Mr 32,000 protein with inhibin activity from porcine follicular fluid. Proc Natl Acad Sci USA 1985;82:7217–7221
Tsonis CG, Sharpe RM: Dual gonadal control of follicle stimulating hormone. Nature 1986;321:724–725
Biscak T, Cajander SB, Vale W, Hsueh AJW: Inhibin: Studies of stored and secreted forms by biosynthetic labelling and immuno detection in cultured rat granulosa cells. Endocrinology 1988;122:741–748
Wrathall JHM, Knight PG: Production of immunoreactive inhibin by bovine granulosa cells in serum free culture: Effects of exogenous steroids and FSH. Domest Anim Endocrinol 1993;10:289–304
Mason AJ, Niall HD, Seeburgh PH: Structure of human ovarian inhibins. Biochem Biophys Res Commun 1986;135:957–964
Davis SR, Dench F, Nikolaidis L, Clements JA, Forage RG, Krozowski Z, Burger HG: Inhibin-A subunit gene expression in the ovaries of immature female rats is stimulated by pregnant mare serum gonadotropin. Biochem Biophys Res Commun 1986;138:1191–1195
Hillier SG, Wickings EJ, Illingworth PI, Yong EL, Reichert LE, Baird DT, McNeilly AS: Control of immunoreactive inhibin production by human granulosa cells. Clin Endocrinol 1991;35:71–78
Tsonis CG, Hillier SG, Baird DT: production of inhibin bioactivity by human-granulosa-lutein cells: Stimulation by LH and testosterone in vitro. J Endocrinol 1987;112:R11–R14
Groome NP, Illingworth PJ, O'Brien M, Cooke I, Ganesan TS, Baird DT, McNeilly AS: Detection of dimeric inhibin throughout the human menstrual cycle by two-site enzyme immunoassay. Clin Endocrinol 1994;40:717–723
Muttukrishna S, Fowler PA, Groome NP, Mitchell GG, Robertson WR, Knight PG: Serum concentrations of dimeric inhibin during the spontaneous humanmenstrual cycle and after treatment with exogenous gonadotrophin. Hum Reprod 1994;9:1634–1642
Groome NP, Illingworth PJ, O'Brien M, Pai R, Rodger FE, Mather J, McNeilly AS: Measurement of dimeric inhibin-B throughout the human menstrual cycle. J Clin Endocrinol Metab 1996;81:1401–1405
Groome NP, Illingworth PJ, O'Brien M, Priddle J, Weaver K, McNeilly AS: Quantification of inhibin pro-αC-containing forms in human serum by a new ultrasensitive two-site enzyme-linked immunosorbent assay. J Clin Endocrinol Metab 1995;80:2926–2932
Knight PG, Muttukrishna S, Groome NP: Development and application of a two-site enzyme immunoassay for the determination of “total” activin A concentrations in serum and follicular fluid. J Endocrinol 1996;148:267–279
Muttukrishna S, Fowler PA, George L, Groome NP, Knight PG: Changes in peripheral serum levels of total activin A during human menstrual cycle and pregnancy. J Clin Endocrinol Metab 1996;81:3328–3334
Lockwood GM, Muttukrishna S, Groome NP, Knight PG, Ledger WL: Circulating inhibins and activin A during GnRH-analogue down-regulation and ovarian hyperstimulation with recombinant FSH for in vitro fertilization and embryo transfer. Clin Endocrinol 1996;45:741–748
Schipper I, Fauser BCJM, van Gaver EBO, Zarutskie PW, Dahl KD: Development of a human granulosa cell culture model with follicle stimulating hormone responsiveness. Hum Reprod 1993;8:1380–1386
Seifer DB, Gardiner AC, Lambert-Messerlian G, Schneyer AL. Differential secretion of dimeric inhibin in cultured luteinized granulosa cells as a function of ovarian reserve. J Clin Endocrinol Metab 1996;81:736–739
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Muttukrishna, S., Groome, N. & Ledger, W. Gonadotropic Control of Secretion of Dimeric Inhibins and Activin A by Human Granulosa–Luteal Cells In Vitro. J Assist Reprod Genet 14, 566–574 (1997). https://doi.org/10.1023/A:1022524516824
Issue Date:
DOI: https://doi.org/10.1023/A:1022524516824