Abstract
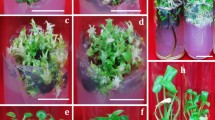
Genotypic differences between six genotypes of Primula vulgaris could be observed in callus induction rate, type of callus, root formation during the callus phase, and shoot regeneration rate. The shoot regeneration rate ranged from zero to 11.6 shoots per explant. There was no correlation between callus induction rate and shoot regeneration rate. Callus consistency and colour were an indicator of the organogenetic capacity of callus. An experiment with different periods of treatment with 4.0 mg l 2,4-dichlorophenoxyacetic acid and 2.0 mg l21 thidiazuron revealed that the shoot regeneration rate varied tremendously between genotypes. In two genotypes a period of 8 weeks on medium with plant growth regulators was sufficient to induce shoot regeneration. In three other genotypes a longer induction period was not able to overcome low regeneration capacity. However an increase in shoot regeneration rate was observed after 16iV32 weeks of induction. Phenotypic stability was also strongly dependent on genotype. In three genotypes the majority of regenerated plants looked normal and were diploid. Aberrations like abnormal growth habit, crinkly leaves, deviation of flower colour or lack of pollen formation occurred in only one genotype at a very low frequency (1.5 genotypes between 12.5 and 18.1 regenerants was tetraploid.
Similar content being viewed by others
References
Ampomah-Dwamena C, Connor AJ & Fautrier AG (1997) Genotypic response of lettuce cotyledons regeneration in vitro. Sci. Hortic. 71: 137-145
Bhagwat B, Vieira LGE & Erickson LR (1996) Stimulation of in vitro shoot proliferation from nodal explants of cassava by thidiazuron, benzyladenine and gibberellic acid. Plant Cell Tiss. Org. Cult. 46: 1-7
Bayliss MW (1980) Chromosomal variation in plant tissue in culture. Int. Rev. Cytology Suppl. 11A: 113-144
Carvalho CHS, Bohorova N, Bordallo PN, Abreu LL, Valicente FH, Bressan W & Paiva E (1997) Type II callus production and plant regeneration in tropical maize genotypes. Plant Cell Rep. 17: 73-76
Coumans M, Coumans-Gillès M-F, Delhez J & Gaspar T (1979) Mass propagation of Primula obconica. Acta Hort. 91: 287-293
D'Amato F (1985) Cytogenetics of plant cell and tissue cultures and their regenerates. Crit. Rev. Plant Sci. 3: 73-112
Gamborg OL, Miller RA & Ojima K (1968) Nutrient requirement of suspension cultures of soybean root cells. Exp. Cell Res. 50: 151-158
Geier T (1991) Chromosome variability in callus produced plants. In: Harding J, Singh F & Mol JNM (eds) Genetics and Breeding of Ornamental Species (pp 79-106). Kluwer Academic Publishers, Dordrecht, The Netherlands
Henry Y, Vain P & De Buyser J (1994) Genetic analysis of in vitro plant tissue culture response and regeneration capacities. Euphytica 79: 45-58
Hutchinson MJ & Saxena PK (1996) Acetylsalicyclic acid enhances and synchronizes thidiazuron-induced somatic embryogenesis in geranium (Pelargonium3hortorum Bailey) tissue cultures. Plant Cell Rep. 15: 512-515
Malik KA & Saxena PK (1992) Regeneration in Phaseolus vulgaris L.: High-frequency induction of direct shoot formation in intact 6 seedling by N6-benzylaminopurine and thidiazurone. Planta 186: 384-389
Merkle S & Götz EM (1990) Micropropagation of inbred lines of Primula acaulis for breeding purposes. Abstracts VIIth International Congress on Plant Tissue and Cell Culture, Amsterdam, June 1990: 116.
Mizuhiro M, Kenichi Y, Ito K, Kadowaki S, Ohashi H & Mii M (2001) Plant regeneration from cell suspension-derived protoplasts of Primula malacoides and Primula obconica. Plant Sci. 160: 1221-1228
Mok MC & Mok DWS (1977) Genotypic response to auxins in tissue cultures of Phaseolus. Physiol. Plant 40: 261-264
Murashige T & Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Phys. Plant. 15: 473-497
Orlikowska T, Nowak E, Marasek A & Kucharska D (1999) Effects of growth regulators and incubation period on in vitro regeneration of adventitious shoots from gerbera petioles. Plant Cell Tiss. Org. Cult. 59: 95-102
Özgen M, Tϋret M, Altinok S & Sancak C (1998) Efficient callus induction and plant regeneration from mature embryo culture of winter wheat (Triticum aestivum L.) genotypes. Plant Cell Rep. 18: 331-335
Pellegrineschi A (1997) In vitro plant regeneration via organogenesis of cowpea [Vigna unguiculata (L.) Walp.]. Plant Cell Rep. 17: 89-95
Schween G & Schwenkel H-G (2002) In vitro regeneration in Primula ssp. via organogenesis. Plant Cell Rep. 20: 1006-1010
Statistisches Jahrbuch (1992) Land-und Forstwissenschaft, Fischerei - Fachserie 3, Reihe 3.1.6. - Bodennutzung - Anbau von Zierpflanzen, Bundesministerium für Ernährung, Landwirtschaft und Forsten, Landwirtschaftsverlag, Münster-Hiltrup (p. 7)
Visser C, Qureshi JA, Gill R & Saxena PK (1992) Morphoregulatory role of thidiazuron. Plant Physiol. 99: 1204-1707
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schween, G., Schwenkel, HG. Effect of genotype on callus induction, shoot regeneration, and phenotypic stability of regenerated plants in the greenhouse of Primula ssp. . Plant Cell, Tissue and Organ Culture 72, 53–61 (2003). https://doi.org/10.1023/A:1021227414880
Issue Date:
DOI: https://doi.org/10.1023/A:1021227414880