Abstract
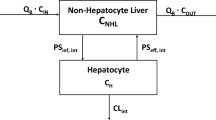
The disposition kinetics of Cyclosporine A (CyA) in rat, based on measurement in arterial blood, appeared dose-linear over a wide iv dose range (1.2–30mg/kg). Physiologically based pharmacokinetic (PBPK) analysis, however, demonstrated that this was an apparent observation resulting from counterbalancing nonlinear factors, such as saturable blood and tissue distribution, as well as clearance (CLb ). A PBPK model was successfully developed taking into account these multiple nonlinear factors. Tissue distribution was distinctly different among various organs, being best described by either a linear model (muscle, fat; Model 1), one involving instantaneous saturation (lung, heart, bone, skin, thymus; Model 2), noninstantaneous saturation (kidney, spleen, liver, gut; Model 3), or one with saturable efflux (brain; Model 4). Overall, the whole body volume of distribution at steady state for unbound CyA (Vuss ) decreased with increasing dose, due at least in part to saturation of tissue-cellular cyclophilin binding. Clearance, essentially hepatic, and described by the well-stirred model, was also adequately characterized by Michaelis–Menten kinetics, Km 0.60 μg/ml. In model-based simulations, both volume of distribution at steady state (V ss,b ) and CLb varied in a similar manner with dose, such that terminal t 1/2 remained apparently unchanged; these dose responses were attenuated by saturable blood binding. CyA concentration measured in arterial blood was not always directly proportional to the true exposure, i.e., unbound or target tissue concentrations. The PBPK model not only described comprehensively such complicated PK relationships but also permitted assessment of the sensitivity of individual parameters to variation in local nonlinear kinetics. Using this approach, dose-dependent CyA uptake into brain was shown to be sensitive to both active and passive transport processes, and not merely the affinity of the active (efflux) transporter at the level of the blood–brain barrier.
Similar content being viewed by others
REFERENCES
L. D. Bower. Therapeutic monitoring for cyclosporine: difficulties in establishing a therapeutic window. Clin. Biochem. 24:81–87 (1991).
B. Legg and M. Rowland, Cyclosporin: erythrocyte binding and an examination of its use to estimate unbound concentrations. Ther. Drug. Monit. 10:16–19 (1988).
R. Kawai and M. Lemaire. Role of blood cell uptake on cyclosporin pharmacokinetics. In P. Tillement and H. Eckert (eds.), Proceeding of the International Symposium on Blood Binding and Drug Transfer, EFC Publishing, Paris, 1993, pp. 89–108.
A. Bernareggi and M. Rowland. Physiological modeling of cyclosporin kinetics in rat and man. J. Pharmacokin. Biopharm. 19:21–50 (1991).
R. Kawai, D. Mathew, C. Tanaka, and M. Rowland. Physiologically-based pharmacokinetics of Cyclosporine A: Extension to tissue distribution kinetics in rat and scale-up to human. J. Pharmacol. Exp. Ther. 287:457–468 (1998).
C. Tanaka, R. Kawai, and M. Rowland. Dose-dependent pharmacokinetics of Cyclosporine A in rat: Events in tissues. Drug Metab. Dispos. 28:582–589 (2000).
R. Kawai, M. Lemaire, J. L. Steimer, A. Bruelisauer, W. Niederberger, and M. Rowland. Physiologically based pharmacokinetic study on a cyclosporin derivative, SDZ IMM 125. J. Pharmacokin. Biopharm. 22:327–365 (1994).
M. Lemaire and P. Tillement. Role of lipoproteins and erythrocytes in the in vitro binding and distribution of cyclosporin A in the blood. J. Pharm. Pharmacol. 34:715–718 (1982).
C. Sloop, L. Dory, and P. Roheim. Interstitial fluid lipoproteins. J. Lipid Res. 28:225–237 (1987).
S. Pang and M. Rowland. Hepatic clearance of drugs. I. Theoretical considerations of a “well-stirred” model and a “parallel tube” model. Influence of hepatic blood flow, plasma and blood cell binding and hepatocellular enzymatic activity on hepatic drug clearance. J. Pharmacokin. Biopharm. 5:625–653 (1977).
M. Rowland and T. N. Tozer. Clinical Pharmacokinetics: Concepts and Applications. 3rd ed., Williams and Wilkins, Philadelphia, 1995, pp. 313–335, 485–489.
H. Akaike. An information criterion (AIC). Math. Sci. 14(153):5–9 (1976).
A. Sakata, I. Tamai, K. Kawazu, Y. Deguchi, T. Ohnishi, A. Saheki, and A. Tsuji. In vivo evidence for ATP-dependent and P-glycoprotein-mediated transport of Cyclosporin A at the blood brain barrier. Biochem. Pharmacol. 48:1989–1992 (1994).
A. Shirai, M. Naito, T. Tatsuta, J. Dong, K. Hanaoka, K. Mikami, T. Oh-hara, and T. Tsuruo. Transport of cyclosporin A across the brain capillary endothelial cell monolayer by P-glycoprotein. Biochim. Biophys. Acta 1222:400–404 (1994).
A. Tsuji, I. Tamai, A. Sakata, Y. Tenda, and T. Terasaki. Restricted transport of cyclosporin A across the blood-brain barrier by a multidrug transporter, P-glycoprotein. Biochem. Pharmacol. 46:1096–1099 (1993).
W. F. Ebling, D. R. Wada, and D. R. Stanski. From piecewise to full physiologic pharmacokinetic modeling: Applied to thiopental disposition in the rat. J. Pharmacokin. Biopharm. 22:259–292 (1994).
S. Bjorkman, D. R. Wada, D. R. Stanski, and W. F. Ebling. Comparative physiological pharmacokinetics of fentanyl and alfentanil in rats and humans based on parametric singletissue models. J. Pharmacokin. Biopharm. 22:381–410 (1994).
G. M. Blakey, I. A. Nestorov, P. A. Arundel, L. J. Aarons, and M. Rowland. Quantitative structure-pharmacokinetics relationships: I. Development of a whole-body physiologically based model to characterize changes in the pharmacokinetics across a homologous series of barbiturates in the rat. J. Pharmacokin. Biopharm. 25:277–312 (1997).
S. Song, H. Suzuki, R. Kawai, C. Tanaka, I. Akasaka, and Y. Sugiyama. Dose-dependent effects of PSC 833 on its tissue distribution and on the biliary excretion of endogenous substrates in rats. Drug Metab. Dispos. 26:1128–1133 (1998).
J. A. Fairley. Intracellular targets of cyclosporine. J. Am. Acad. Dermatol. 23:1329–1334 (1990).
M. L. McDonald, T. Ardito, W. H. Marks, M. Kashgarian, and H. Lorber. The effect of cyclosporine administration on the cellular distribution and content of cyclophilin. Transplantation 53:460–466 (1992).
A. Vickers, V. Fischer, S. Connors, R. Fischer, J. P. Baldeck, G. Maurer, and K. Brendel. Cyclosporin A metabolism in human liver, kidney, and intestine slices: Comparison to rat and dog slices and human cell lines. Drug Metab. Dispos. 20:802–809 (1992).
B. M. J. Foxwell, A. Mackie, V. Ling, and B. Ryffel. Identification of the multidrug-resistance related P-glycoprotein as a cyclosporine binding protein. Mol. Pharmacol. 36:543–546 (1988).
D. C. Dalgarno, M. W. Harding, A. Lazarides, R. Handschumacher, and I. M. Armitage. 1H NMR studies on bovine cyclophilin: Preliminary structural characterization of this specific cyclosporin A binding protein. Biochemistry 25:6778 (1986).
B. Ryffel. Cyclosporin binding proteins. Biochem. Pharmacol. 46:1–12 (1993).
P. C. De Groen, A. J. Aksamit, J. Rakela, G. S. Forbes, and R. Krom. Central nervous system toxicity after liver transplantation. The role of cyclosporine and cholesterol. New Engl. J. Med. 317:861–866 (1987).
M. Lemaire, A. Bruelisauer, P. Guntz, and H. Sato. Dose-dependent brain penetration of SDZ PSC 833, a novel multidrug resistance-reversing cyclosporin, in rats. Cancer Chemother. Pharmacol. 38:481–486 (1996).
L. C. Racusen, L. M. Famiglio, B. A. Fivush, D. S. Olton, and K. Solez. Neurological abnormalities and mortality in rats treated with cyclosporine A. In B. D. Kahan (ed.), Cyclosporine: Therapeutic use in transplantation. Transplantation Proceedings Reprint (June Suppl. 3), Grune and Stratton Inc., New York, 1988, pp. 934–936.
A. H. Schinkel, E. Wagenaar, L. V. Deemter, C. A. A. M. Mol, and P. Borst. Absence of the mdrla P-glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J. Clin. Invest. 96:1698–1705 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tanaka, C., Kawai, R. & Rowland, M. Physiologically Based Pharmacokinetics of Cyclosporine A: Reevaluation of Dose–Nonlinear Kinetics in Rats. J Pharmacokinet Pharmacodyn 27, 597–623 (1999). https://doi.org/10.1023/A:1020978509566
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1020978509566