Abstract
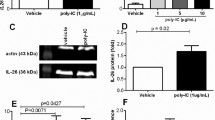
Cytokine networks are important in regulating the traffic of inflammatory cells in the airways. Interleukin-8 (IL-8) released by human bronchial epithelial cells (HBECs) is thought to be of particular importance in attracting neutrophils and monocytes to sites of inflammation. Increased release of IL-8 by HBECs in response to Th-1 cytokines such as TNF alpha and IL-1 beta may be an important pathophysiologic pathway. The present study was designed to explore the role of the Th2 cytokine IL-4 and the functionally related interleukins IL-10, and IL-13 on the regulation of IL-8 release by HBECs. HBECs (passage 4–6) were cultured in LHC9/RPMI and when confluent cells were stimulated in unsupplemented medium LHCD/RPMI by IL-4, IL-10, and IL-13 at 10 ng/ml concentration for all cytokines. TNF alpha stimulation was used as a positive control. After 24 hours supernatants were collected and tested for IL-8 by a sandwich ELISA. Unstimulated HBECs spontaneously released limited amounts of IL-8 (11 ± 1 pM) and significantly increased cytokine production in response to IL-4 (42 ± 1 pM), IL-13 (30 ± 1 pM) and TNF (128 ± 11 pM). Stimulation with IL-10 (11 ± 1 pM) did not change basal production of IL-8. When HBECs were co-stimulated with IL-4 plus TNF, the production of IL-8 was further increased (204 ± 5 pM). In contrast, IL-10 attenuated the effect of TNF during co-stimulation (82 ± 5 pM). IL-13 did not affect the release of IL-8 induced by TNF (111 ± 9 pM). Northern blot analysis of IL-8 mRNA levels showed the highest induction of IL-8 mRNA in HBECs co-stimulated with TNF and IL-4. We conclude from our study that IL-4 directly induces IL-8 release from HBECs and amplifies the release of IL-8 in response to TNF alpha. IL-13 is less active and IL-10 has an inhibitory effect. Airway epithelial cells are able to interact, therefore, with products of both Th1 and Th2 cells with respect to modulating release of IL-8.
Similar content being viewed by others
REFERENCES
Raeburn, D. and S. E. Webber. 1994. Proinflammatory potential of the airway epithelium in bronchial asthma. Eur. Respir. J. 7:2226-2233.
Rennard, S. I., D. J. Romberger, J. H. Sissons, S. G. von Essen, I. Rubinstein, R. A. Robbins, and J. R. Spurzem. 1994. Airway epithelial cells: functional roles in airway disease. Am. J. Respir. Crit. Care Med. 150:527-530.
Strieter, R. M., N. W. Lukacs, T. J. Standiford, and S. L. Kunkel. 1993. Cytokines and lung inflammation: mechanisms of neutrophil recruitment to the lung. Thorax 48:765-769.
Baggiolini, M., P. Imboden, and P. Detmers. 1992. Neutrophil activation and the effects of interleukin-8/neutrophil-activating peptide 1 (IL-8/NAP-1). In: Baggiolini, M., Sorg, C., eds. Interleukin-8 (NAP-1) and related chemotactic cytokines. Basel (Switzerland): Karger, pp. 1-17.
Detmers, P. A., S. K. Lo, E. Olsen-Egbert, A. Walz, M. Baggiolini, and Z. A. Cohn. 1990. Neutrophil-activating protein 1/interleukin-8 stimulates the binding activity of the leukocyte adhesion receptor CD11b/CD18 on human neutrophils. J. Exp. Med. 171:1155-1162.
Wymann, M. P., P. Kernen, D. A. Deranleau, B. Dewald, V. von Tscharner, and M. Baggiolini. 1987. Oscillatory motion in human neutrophils responding to chemotactic stimuli. Biochem. Biophys. Res. Commun. 147:361-368.
Leonard, E. J., T. Yoshimura, S. Tanaka, and M. Raffeld. 1991. Neutrophil recruitment by intradermally injected neutrophil attractant/activating protein-1 (NAP-1) in human subjects. J. Invest. Dermatol. 96:690-694.
Miller, E. J., A. B. Cohen, S. Nagao, D. Griffith, R. J. Maunder, T. R. Martin, J. P. Weiner-Kronish, M. Sticherling, E. Christophers, and M. A. Matthay. 1992. Elevated levels of NAP-1/Interleukin-8 are present in the airspaces of patients with the adult respiratory distress syndrome and are associated with increased mortality. Am. Rev. Respir. Dis. 146:427-432.
Car, B. D., F. Meloni, M. Luisetti, G. Semenzato, G. Gialdroni-Grassi, and A. Walz. 1994. Elevated IL-8 and MCP-1 in the bronchoalveolar lavage fluid of patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am. J. Respir. Crit. Care Med. 149:655-659.
Konno, S., Y. Gonokami, M. Kurokawa, K. Kawazu, K. Asano, K. Okamoto, and M. Adachi. 1996. Cytokine concentrations in sputum of asthmatic patients. Int. Arch. Allergy Immunol. 109:73-78.
Levine, S. J., P. Larivee, C. Logun, C. W. Angus, and J. H. Shelhamer. 1993. Corticosteroids differentially regulate secretion of IL-6, IL-8, and G-CSF by a human bronchial epithelial cell line. Am. J. Physiol. 265:L360-L368.
Vittori, E., F. Sciacca, F. Colotta, A. Mantovani, and S. Mattoli. 1992. Protective effect of nedocromil sodium on the interleukin-1 induced production of interleukin-8 in human bronchial epithelial cells. J. Allergy Clin. Immunol. 90:76-84.
Romagnani, S. 1995. Biology of human Th1 and Th2 cells. J. Clin. Immunol. 15:121-129.
Maestrelli, P., A. Di Stefano, P. Occari, G. Turato, G. Milani, F. Pivirotto, C. E. Mapp, L. M. Fabbri, and M. Saetta. 1995. Cytokines in the airway mucosa of subjects with asthma induced by toluene diisocyanate. Am. J. Respir. Crit. Care Med. 151:607-612.
Umetsu, D. T. and R. H. De Kruyff. 1997. Th1 and Th2 CD4+ cells in human allergic diseases. J. Allergy Clin. Immunol. 100:1-6.
Swain, S. L. 1994. IL-4 dictates T-cell differentiation. Res. Immunol. 144:616-620.
O'Garra, A., S. E. Macatonia, C. S. Hsieh, and K. M. Murphy. 1994. Regulatory role of IL-4 and other cytokines in T helper cell development in an abTCR transgenic mouse system. Res. Immunol. 144:620-625.
Paul, W. E. 1992. The role of IL-4 in the regulation of B cell development, growth, and differentiation. In: Spits, H., ed. IL-4: Structure and Function. CRC Press, Boca Raton, Florida: pp. 58-73.
De Waal Melafyt, R., H. Yssel, M.-G. Roncarolo, H. Spits, and J. E. De Vries. 1992. Interleukin-10. Curr. Opinion. Immun. 314-320.
Moore, K. W., A. O'Garra, R. De Waal Malefyt, P. Vierra, and T. R. Mosmann, 1993. Interleukin-10. Ann. Rev. Immunol. 11:165-190.
Pretolani, M. and M. Goldman. 1997. IL-10: a potential therapy for allergic inflammation. Immunol. Today 18:277-280.
Fiorentino, D. F., A. Zlotnik, T. R. Mosmann, M. Howard, and A. O'Gara. 1991. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 147:3815-3822.
Casatella, M. A., L. Meda, S. Bonora, M. Ceska, and G. Constantini. 1993. Interleukin-10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J. Exp. Med. 178:2207-2211.
Mosmann, T. R. 1994. Interleukin-10. In: Thompson, A. W., ed. The Cytokine Handbook. Academic Press, San Diego, California: pp. 223-237.
Zurawski, G. and J. E. De Vries. 1994. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol. Today 15:19-26.
Zurawski, S. M., F. Vega Jr., B. Huyghe, and G. Zurawski. 1993. Receptors for interleukin-13 and interleukin-4 are complex and share a novel component that functions in signal transduction. EMBO J. 12:2663-2670.
Kelsen, S. G., I. A. Mardini, S. Zhou, J. L. Benovic, and N. C. Higgins. 1992. A technique to harvest viable tracheobronchial epithelial cells from living human donors. Am. J. Respir. Cell Mol. Biol. 7:66-72.
Beckman, J. D., H. Takizawa, D. Romberger, M. Illig, L. Classen, K. Rickard, and S. I. Rennard. 1992. Serum-free culture of fractionated bovine bronchial epithelial cells. In: Vitro Cell. Dev. Biol. 28A:39-46.
Boyce, S. T., and R. G. Ham. 1985. Cultivation, frozen storage, and clonal growth of normal epidermal keratinocytes in serum free media. J. Tissue Cult. Methods 9:83-93.
Chomczynski, P. and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analyt. Biochem. 162:156-159.
Kwon, O. J., T. Bo, P. D. Collins, I. M. Adcock, J. C. Mak, R. R. Robbins, K. F. Chung, and P. J. Barnes. 1994. Tumor necrosis factor-induced interleukin-8 expression in cultured human airway epithelial cells. Am. J. Physiol. 267:L398-L405.
Robbins, R. A., P. J. Barnes, D. R. Springall, J. B. Warren, O. J. Kwon, L. D. K. Buttery, A. J. Wilson, D. A. Geller, and J. M. Polak. 1994. Expression of inducible nitric oxide synthase in human lung epithelial cells. Biochem. Biophys. Res. Commun. 203:209-218.
Colgan, S. P., M. B. Resnick, C. A. Parkos, C. Delp-Archer, D. McGuirk, A. E. Bacarra, P. F. Weller, and J. L. Madara. 1994. IL-4 directly modulates function of a model human intestinal epithelium. J. Immunol. 153:2122-2129.
Postlethwaite, A. E. and J. M. Seyer. 1991. Fibroblast chemotaxis induction by a recombinant IL-4. J. Clin. Invest. 87:1240.
Piela-Smith, T. H., G. Broketa, A. Hand, and J. H. Korn. 1992. Regulation of ICAM-1 expression and function in human dermal fibroblasts by IL-4. J. Immunol. 148:1375-1381.
Leung, K. N., N. K. Mak, M. C. Fung, and A. J. Hapel. 1994. Synergistic effect of IL-4 and TNF-alpha in the induction of monocytic differentiation of a mouse myeloid leukaemic cell line (WEHI-3B JCS). Immunology 81:65-72.
Cromwell, O., Q. Hamid, C. J. Corrigan, J. Barkans, Q. Meng, P. D. Collins, and A. B. Kay. 1992. Expression and generation of interleukin-8, IL-6 and granulocyte-macrophage colony-stimulating factor by bronchial epithelial cells and enhancement by IL-1 beta and tumor necrosis factor-alpha. Immunology 77:330-337.
Erger, R. A., and T. B. Casale. 1995. Interleukin-8 is a potent mediator of eosinophil chemotaxis through endothelium and epithelium. Am. J. Physiol. 268:L117-L122.
Mio, T., D. J. Romberger, A. B. Thompson, R. A. Robbins, A. Heires, and S. I. Rennard. 1997. Cigarette smoke induces interleukin-8 release from human bronchial epithelial cells. Am. J. Respir. Crit. Care Med. 155:1770-1776.
Adachi, Y., T. Mio, K. Takigawa, I. Striz, D. J. Romberger, R. A. Robbins, J. R. Spurzem, P. Heires, and S. I. Rennard. 1997. Mutual inhibition by TGF-b and IL-4 in cultured human bronchial epithelial cells. Am. J. Physiol. 273:L701-L708.
Marone, G. 1998. Asthma: recent advances. Immunol. Today 19:5-9.
Massion, P. P., H. Inoue, J. Richman-Eisenstat, D. Gruneberger, P. G. Jorens, B. Housset, J. F. Pittet, J. P. Wiener-Kronish, and J. A. Nadel. 1994. Novel Pseudomonas product stimulates interleukin-8 production in airway epithelial cells in vitro. J. Clin. Invest. 93:26-32.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stříž, I., Mio, T., Adachi, Y. et al. IL-4 and IL-13 Stimulate Human Bronchial Epithelial Cells to Release IL-8. Inflammation 23, 545–555 (1999). https://doi.org/10.1023/A:1020242523697
Issue Date:
DOI: https://doi.org/10.1023/A:1020242523697