Abstract
Purpose. 1. To determine properties of the estimated variance component for the subject-by-formulation interaction (σ2 D) in investigations of individual bioequivalence (IBE), and 2. to evaluate the prevalence of interactions in replicate-design studies published by FDA.
Methods. Four-period crossover studies evaluating IBE were simulated repeatedly. Generally, the true bioequivalence of the two formulations, including σ2 D= 0, was assumed, σ2 D was then estimated in a linear mixed-effect model by restricted maximum likelihood (REML). The same method was applied for estimating σ2 D for the data sets of FDA.
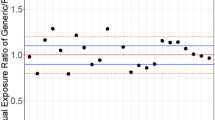
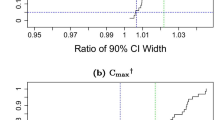
Results. 1.σD estimated by REML was positively biased. The bias and dispersion of the estimated σDincreased approximately linearly with the estimated within-subject standard deviation for the reference formulation (σWR). Only a small proportion of the estimated σD exceeded the estimated σWR. 2. Distributions of the estimated σD were evaluated. At σWR = 0.30, a level of estimated σD= 0.15 was exceeded, by random chance, with a probability of about 25%. 3. Importantly, the behaviour of the σ2 D values estimated from the FDA data sets was similar to that exhibited by the simulated estimates of σ2 D which were generated under the conditions of true bioequivalence.
Conclusions. 1. σD estimated by REML is biased; the bias increases proportionately with the estimated σWR. Consequently, exceeding a fixed level of σD (e.g., 0.15) does not indicate substantial interaction. 2. The data sets of FDA are compatible with the hypothesis of σ2 D = 0. Consequently, they do not demonstrate the prevalence of subject-by-formulation interaction. Therefore, it could be sufficient and reasonable to evaluate bioequivalence from 2-period crossover studies.
Similar content being viewed by others
REFERENCES
S. Anderson and W. W. Hauck. Considerations of individual bioequivalence. J. Pharmacokin. Biopharm. 18:259–273 (1993).
R. Schall and R. L. Williams. Towards a practical strategy for assessing individual bioequivalence. J. Pharmacokin. Biopharm. 24:133–149 (1996).
M. L. Chen. Individual bioequivalence — a regulatory update. J. Biopharm. Stat. 7:5–11 (1997).
R. N. Patnaik, L. J. Lesko, M.-L. Chen, and R. L. Williams. Individual bioequivalence—new concepts in the statistical assessment of bioequivalence metrics. Clin. Pharmacokin. 33:1–6 (1997).
FDA. In Vivo Bioequivalence Studies Based on Population and Individual Bioequivalence Approaches—Draft Guidance for Industry. Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Rockville, MD, October, 1997.
FDA. Bioequivalence studies: bioequivalence data. Published on Internet: www.fda.gov/cder/bioequivdata/index.htm. (1998).
R. Schall and H. E. Luus. On population and individual bioequivalence. Stat. Med. 12:1109–1124 (1993).
B. Efron and R. J. Tibshirani. An Introduction to the Bootstrap. Chapman and Hall, New York (1993).
FDA. Statistical methods for obtaining confidence intervals for individual and population bioequivalence criteria: technical summary. Published on Internet: www.fda.gov/cder/bioequivdata/statproc.htm. (1998).
L. Endrenyi, G. L. Amidon, K. K. Midha, and J. P. Skelly. Individual bioequivalence: attractive in principle, difficult in practice. Pharm. Res. 15:1321–1325 (1998).
A. W. Boddy, F. C. Snikeris, R. O. Kringle, G. C. G. Wei, J. A. Opperman, and K. K. Midha. An approach for widening the bioequivalence limits in the case of highly variable drugs. Pharm. Res. 12:1865–1868 (1995).
L. Endrenyi and Y. Hao. Asymmetry of the mean-variability tradeoff raises questions about the model in investigations of bioequivalence. Int. J. Clin. Pharmacol. Ther. 36:450–457 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Endrenyi, L., Tothfalusi, L. Subject-by-Formulation Interaction in Determinations of Individual Bioequivalence: Bias and Prevalence. Pharm Res 16, 186–190 (1999). https://doi.org/10.1023/A:1018899504711
Issue Date:
DOI: https://doi.org/10.1023/A:1018899504711