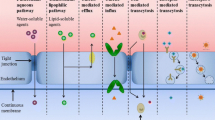
Abstract
Purpose. Appropriate physicochemical parameters are desired for the prediction of passive intestinal drug absorption during lead compound selection and drug development.
Methods. Liposome distribution coefficients measured titrimetrically and solubility data at pH 6.8 were used to characterize 21 structurally diverse ionizable drugs covering a range from <5% to almost complete absorption.
Results. A sigmoidal relationship was found between the percentage of human passive intestinal absorption and a new absorption potential parameter calculated from liposome distribution data and the solubility dose ratio. In contrast, the human absorption data did not correlate with an octanol-based absorption potential or partitioning data alone. Poor correlations were found between liposome and octanol partitioning of ionic species or nonionic bases indicating the profound differences of the partitioning systems.
Conclusions. Liposome distribution coefficients of ionizable drugs derived by a pH-metric titration were successfully used to calculate a parameter that correlates with the percentage of passive intestinal absorption in humans. Profound differences between liposome and octanol partitioning were found for a highly diverse set of species. This titration technique may serve to generate liposome partitioning data for the selection and optimization of lead compounds and in drug development.
Similar content being viewed by others
REFERENCES
C. A. Lipinski, F. Lombardo, B. W. Dominy, and P. J. Feeney. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Del. Rev. 23:3–25 (1997).
S. Yee. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man—fact or myth. Pharm. Res. 14:763–766 (1997).
K. J. Schaper. Absorption of ionizable drugs: nonlinear dependence on logP, pKa and pH-quantitative relationships. Quant.Struct.-Act.Relat. 1:13–27 (1982).
P. A. Shore, B. B. Brodie, and C. A. M. Hogben. The gastric secretion of drugs: A pH partition hypothesis. J. Pharmacol. Exp. Ther. 119:361–369 (1957).
C. J. Alcorn, R. J. Simpson, D. E. Leahy, and T. J. Peters. Partition and distribution coefficients of solutes and drugs in brush border membrane vesicles. Biochem. Pharmacol. 45:1775–1782 (1993).
F. Barbato, M. I. la Rotonda, and F. Quaglia. Interactions of nonsteroidal antiinflammatory drugs with phospholipids: comparison between octanol/buffer partition coefficients and chromatographic indexes on immobilized artificial membranes. J. Pharm. Sci. 86:225–229 (1997).
H.-Y. Cheng, C. S. Randall, W. W. Holl, P. P. Constantinides, T.-L. Yue, and G. Z. Feuerstein. Carvedilol-liposome interaction: evidence for strong association with the hydrophobic region of the lipid bilayers. Biochim. Biophys. Acta 1284:20–28 (1996).
G. V. Betageri, Y. Theriault, and J. A. Rogers. NMR Study of the interaction of beta-blockers with sonicated dimyritoylphos-phatidylcholine liposomes in the presence of praseodymium cation. Membrane Biochem. 8:197–206 (1989).
F. A. P. C. Gobas, J. M. Lahittete, G. Garofalo, W. Y. Shiu, and D. MacKay. A novel method for measuring membrane-water partition coefficients of hydrophobic organic chemicals: comparison with 1-octanol-water partitioning. J. Pharm. Sci. 77:265–272 (1988).
A. Avdeef, K. J. Box, J. E. A. Comer, C. Hibbert, and K. Y. Tam. pH-metric logP 10: Determination of vesicle membrane-water partition coefficients of ionisable drugs. Pharm. Res. 15:209–215 (1998).
A. Avdeef. pH-Metric logP. Part 1: Difference plots for determining ion-pair octanol-water partition coefficients of multiprotic substances. Quant. Struct.-Act. Relat. 11:510–517 (1992).
A. Avdeef. pH-metric logP-2. Refinement of partition coefficients and ionisation constants of multiprotic substances. J. Pharm. Sci. 82:183–190 (1993).
P. Finholt and S. Solvang. Dissolution kinetics of drugs in human gastric juice—the role of surface tension. J. Pharm. Sci. 57:1322–1326 (1968).
L. S. C. Wan and P. F. S. Lee. CMC of Polysorbates. J. Pharm. Sci. 63:136–137 (1974).
G. Camenisch, G. Folkers, and H. van de Waterbeemd. Review of passive drug absorption models: historical background, recent developments and limitations. Pharm. Acta Helv. 71:309–327 (1996).
J. B. Dressman, G. L. Amidon, and D. Fleisher. Absorption potential: Estimating the fraction absorbed for orally administered compounds. J. Pharm. Sci. 74:588–589 (1985).
J. B. Dressman, and V. A. Gray. Change of pH intestinal fluid. Pharmacopeial Forum 22:1943–1945 (1996).
H. Lennernäs. Does fluid flow across the intestinal mucosa affect quantitative oral drug absorption? Is it time for a reevaluation? Pharm. Res. 12:1573–1582 (1995).
K. Hartke (ed). DAB10 Kommentar Wiss. Verl.-Ges. Stuttgart, 1991.
P. de Miranda, and M. R. Blum. Pharmacokinetics of acyclovir after intravenous and oral administration. J. Antimicrob. Chemother. 12Suppl. B:29–37 (1983).
H. Breithaupt and M. Tittel. Kinetics of allopurinol after single intravenous and oral doses. Eur. J. Clin. Pharmacol. 22:77–84 (1982).
F. von Bruchhausen (ed.). Hagers Handbuch der pharmazeutischen Praxis, 5. Aufl., Springer Verlag Berlin, 1993.
W. F. Frishman. Atenolol and timolol, two new systemic b-adrenoceptor antagonists. N. Engl. J. Med. 306:1456–1462 (1982).
J. E. F. Reynolds, (ed), Martindale, The Extra Pharmacopoeia, 31st Edition, The Royal Pharmaceutical Society London, 1996.
K. Lauritsen, L. S. Laursen, and J. Rask-Madsen. Clinical Pharmacokinetics of drugs used in the treatment of gastrointestinal diseases (Part 1). Clin. Pharmacokinet. 19:11–31 (1990).
L. Lemberger, R. F. Bergstrom, R. L. Wollen, N. A. Farid, G. G. Enas, and G. R. Aronoff. Fluoxetine: Clinical pharmacology and physiological disposition. J. Clin. Psychiatry 46:14–19 (1985).
R. F. N. Mills, S. S. Adams, E. E. Cliffe et al. The metabolism of ibuprofen. Xenobiotica 3:589–598 (1973a).
R. A. Theodor, H.-J. Weimann, W. Weber et al. Absolute bioavailability of moxonidine. Eur. J. Drug Metab. Pharmacokinet. 16:153–159 (1991).
B. Fulton, and K. L. Goa. Olanzapine A review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs 53:281–298 (1997).
P. A. Routledge and D. G. Shand. Clinical pharmacokinetics of propranolol. Clin. Pharmacokinet. 4:73–90 (1979).
M. H. Skinner, and T. F Blaschke. Clinical pharmacokinetics of rifabutin. Clin. Pharmacokinet. 28:115–125 (1995).
J. C. Jensen. Clinical pharmacokinetics of terbinafine. Clin. Exp. Dermatol. 14:110–113 (1989).
H. D. Langtry and D. M. Campoli-Richards. Zidovudin. Drugs 37:407–450 (1989).
A. N. Wadworth and D. McTavish. Zopiclone A review of its pharmacological properties and therapeutic efficacy. Drugs & Aging 3:441–459 (1993).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balon, K., Riebesehl, B.U. & Müller, B.W. Drug Liposome Partitioning as a Tool for the Prediction of Human Passive Intestinal Absorption. Pharm Res 16, 882–888 (1999). https://doi.org/10.1023/A:1018882221008
Issue Date:
DOI: https://doi.org/10.1023/A:1018882221008