Abstract
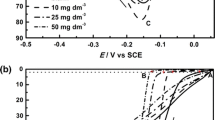
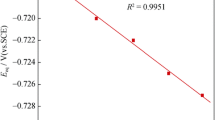
Nickel electrodeposition from 0.2 M formate–chloride solutions is studied. Depending on electrolyte pH0, the highest current density of the electrodeposition of compact nickel deposits varies from 3 (pH03.5) to 40 A dm–2(pH02.0). With the current efficiency for nickel taken into account, this corresponds to nickel deposition rates of 3 to 25 A dm–2. One of the reasons for the high permissible current densities is good buffer properties of the electrolyte. Computer calculations show that the considerable acceleration of the nickel electrodeposition is due to mass transport accelerated by the formation of complex [NiL]+cations. The complex formation also affects the intensity of interaction between nickel and hydrogen ions transported to the cathode. The current by nickel increases due to the participation of formic acid molecules in the hydrogen evolution.
Similar content being viewed by others
REFERENCES
Kudryavtsev, N.T., Gal'vanotekhnika (Electroplating), Moscow: Gizlegprom, 1940.
Kudryavtsev, N.T., Korol'kova, O.M., and Fedurkin, V.V., Zh. Prikl. Khim. (Leningrad), 1949, vol. 22, p. 586.
Kudryavtsev, N.T., Elektroliticheskie pokrytiya metallami (Electroplating with Metals), Moscow: Khimiya, 1979.
Doktorina, S.V. and Kudryavtsev, N.T., Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol., 1960, vol. 3, p. 497.
Kudryavtsev, N.T., Tsupak, T.E., and Przyluski, J., Zashch. Met., 1967, vol. 3, p. 447.
Kudryavtsev, N.T., Tsupak, T.E., Mekhtiev, M.A., and Marchenkov, Yu.M., Zashch. Met., 1977, vol. 13, p. 618.
Kudryavtsev, N.T., Tsupak, T.E., Bud'ko, V.P., and Metkhiev, M.A., Tr. Mosk. Khim.-Tekhnol. Inst. im. D.I. Mendeleeva, 1977, no. 95, p. 42.
Kudryavtsev, N.T., Loseva, E.I., Tsupak, T.E., and Mel'nikov, V.V., Izv. Akad. Nauk Latv. SSR, Ser. Khim., 1980, no. 3, p. 301.
Tsupak, T.E., Bek, R.Yu., Loseva, E.I., and Borodikhina, L.I., Elektrokhimiya, 1982, vol. 18, p. 86.
Bek, R.Yu., Tsupak, T.E., Nguen Zui Shi, and Borodikhina, L.I., Elektrokhimiya, 1985, vol. 21, p. 1346.
Stability Constants of Metal-Ion Complexes. Part B: Organic Ligands, Perrin, D.D., Comp., Canberra: Australian Natl. Univ., 1979, IUPAC Chemical Data Series no. 22, p. 286.
P ibil, R., Komplexony v chemické analyse, Praha: Nakl. eskoslovenske Akademie Ved., 1957.
Bek, R.Yu., Tsupak, T.E., Nguen Zui Shi, and Borodikhina, L.I., Elektrokhimiya, 1985, vol. 21, p. 1190.
Tsupak, T.E., Kopteva, N.I., Bek, R.Yu., and Shuraeva, L.I., Elektroosazhdenie metallov i splavov (Electrodeposition of Metals and Alloys), Moscow: MKhTI, 1991, p. 68.
Ibl, N. and Venczel, J., Metalloberflaeche, 1970, vol. 24, p. 365.
Morris, D.F., Reed, G.L., Stabre, D.N., and Waters, D.N., J. Inorg. Nucl. Chem., 1965, vol. 27, p. 337.
Bek, R.Yu. and Tsupak, T.E., Elektrokhimiya, 1987, vol. 23, p. 560.
Kharkats, Yu.I., J. Electroanal. Chem., 1979, vol. 105, p. 97.
Kharkats, Yu.I., Elektrokhimiya, 1978, vol. 14, p. 1716.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tsupak, T.E., Bek, R.Y., Dzie Wei et al. Role of Complex Formation in Mass Transport during Nickel Electrodeposition from Low-Concentration Formate–Chloride Electrolytes. Russian Journal of Electrochemistry 37, 730–734 (2001). https://doi.org/10.1023/A:1016724919628
Issue Date:
DOI: https://doi.org/10.1023/A:1016724919628