Abstract
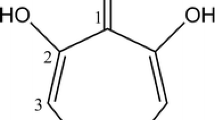
We report here on a new class of siderophores isolated from Rhodococcus erythropolis IGTS8, the first structurally characterized from any species of Rhodococcus and for which we suggest the name heterobactins. These siderophores consist of a tripeptide of sequence (N-OH)-L-Orn-Gly-D-Orn-(δ-N-dihydroyxbenzoate). The alpha amino group of the D-Orn is derivatized either as a 2-hydroxybenzoxazolate in heterobactin A or remains free in heterobactin B. The structures were determined by a combination of amino acid analysis, mass spectrometry and NMR methods. The two new compounds are true siderophores in that they relieve iron limited growth in the producing strain. The heterobactins are also transported by other non-producing bacteria. Growth promotion tests using various transport mutants revealed that in E. coli heterobactin A is only recognized by the catecholate receptor Cir while heterobactin B is taken up in both E. coli and A. flavescens JG9 via a hydroxamate transport system.
Similar content being viewed by others
References
Chipperfield JR, Ratledge C. 2000 Salicylic acid is not a bacterial siderophore: a theoretical study. BioMetals 13, 165–168.
Cohen SM, Meyer M, Raymond KN. 1998 Enterobactin protonation and iron release: Hexadentate tris-salicylate ligands as models for triprotonated ferric enterobactin. J Am Chem Soc 120, 6277–6286.
Drechsel H, Winkelmann G. 1997 Iron chelation and siderophores. In: Winkelmann G and Carrano CJ, eds. Transition Metals in Microbial Metabolism. Amsterdam: Harwood Academic Publishers; 1–49.
Diarra MS, Lavoie MC, Jaques M, Darwish I, Dolence EK, Dolence JA, Gosh A, Gosh M, Miller MJ, Malouin F. 1996 Species selectivity of new siderophore-drug conjugates that use specific iron uptake for entry into bacteria. Antimicrob Agents & Chemother 40, 2610–2617.
Hantke K. 1990 Dihydroxybenzoylserine-a siderophore for E. coli. FEMS Microbiol Lett 67, 5–8.
Kayser KJ, Bielaga-Jones BA, Jackowski K, Odusan O, Kilbane JJ. 1993 Utilization of organosulphur compounds by axenic and mixed cultures of R. rhodochrous IGTS8. J Gen Microbiol 139, 3123–3129.
Komagata K, Suzuki K. 1987 Lipid and cell-wall analysis in bacterial systematics. Methods in Microbiology 19, 161–207.
Lane SJ, Marshall PS, Upton RJ, Ratledge C. 1998 Isolation and characterization of carboxymycobactins as the second extracellular siderophores in Mycobacterium smegmatis. BioMetals 11, 13–20.
Martinez JS, Zhang GP, Holt PD, Jung HT, Carrano CJ, Haygood MG, Butler A. 2000 A New class of self-assembling amphiphilic siderophores from marine bacteria. Science 287, 1245–1246.
Möllmann U, Gosh A, Dolence EK, Dolence JA, Gosh M, Miller MJ, Reisbrodt R. 1998 Selective growth promotion and growth inhibition of Gram-neagtive and Gram-positive bacteria by synthetic siderophore-ß-lactam conjugates. BioMetals 11, 1–12.
Nikaido H, Rosenberg EY. 1990 Cir and Fiu proteins in the outer membrane of Escherichia coli catalyze transport of monomeric catechols: Study with ?-lactam antibiotics containing catechol and analogous groups. J Bacteriol 172, 1361–1367.
Rabsch W, Winkelmann G. 1991 The specificity of bacterial siderophore receptors probed by bioassays. BioMetals 4, 244–250.
Rainey AF, Burghardt J, Kroppenstedt RM, Klatte S, Stackebrandt E. 1995 Phylogenetic analysis of the genera Rhodococcus and Nocardia and evidence for the evolutionary origin of the Nocardia from within the radiation of Rhodococcus species. Microbiology 141, 523–528.
Ratledge C, Patel PV. 1976 The isolation, properties and taxonomic relevance of lipid-soluble, iron-binding compounds (the nocobactins) from Nocardia. J Gen Microbiol 93, 141–152.
Ratledge C, Dover LG. 2000 Iron metabolism in pathogenic bacteria. Annu Rev Microbiol 54, 881–941.
Telford JR, Raymond KN. 1997 Amonabactin: a family of novel siderophores from a pathogenic bacterium. J Biol Inorg Chem 2, 750–761.
Telford JR, Raymond KN. 1998 Coordination chemistry of the amonabactins, bis(catecholate) siderophores from Aeromonas hydrophila. Inorg Chem 37, 4578–4583.
Winkelmann G, Carrano CJ (eds.) 1997 Transition Metals in Mircobial Metabolism. Amsterdam: Harwood Academic Publishers.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Carrano, C.J., Jordan, M., Drechsel, H. et al. Heterobactins: A new class of siderophores from Rhodococcus erythropolis IGTS8 containing both hydroxamate and catecholate donor groups. Biometals 14, 119–125 (2001). https://doi.org/10.1023/A:1016633529461
Issue Date:
DOI: https://doi.org/10.1023/A:1016633529461