Abstract
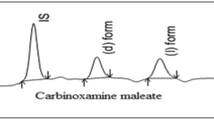
A method is described for the quantification of baclofen enantiomers in biological material (urine, plasma, and cerebrospinal fluid). The samples were extracted by liquid–solid extraction using Sep-Pak CIS cartridges. The subsequent derivatization procedure contained two separate steps. (1) The butyl esters of the enantiomers were formed using butanolic hydrochloric acid (followed by ion-pair extraction of the intermediate products). (2) A chiral derivatization was then performed using S-( + )-naproxen chloride as reagent. S-( + )-Benoxaprofen chloride can also be used. The diastereomeric amides were separated by high-performance liquid chromatography (HPLC) on a silica gel column (mobile phase, n-hexane/dichloromethane/ethanol; detection, fluorescence measurement at 335/365 nm). The described procedure was also used for the quantification of the fluoro analogue of baclofen. Urinary excretion of baclofen enantiomers was investigated in two healthy volunteers after p.o. administration.
Similar content being viewed by others
REFERENCES
N. G. Bowery. Trends Pharmacol. Sci. Oct.:400–403 (1982).
H. R. Olpe, H. Demieville, V. Baltzer, W. L. Bencze, W. P. Koella, P. Wolf, and H. L. Haas. Eur. J. Pharmacol. 52:133–136 (1978).
D. F. Smith and W. H. Pirkle. Psychopharmacology 89:392–393 (1986).
R. P. Weatherby, R. D. Allan, and G. A. R. Johnston. J. Neurosci. Methods 10:23–28 (1984).
E. W. Wuis, E. W. J. Beneken Kolmer, L. E. C. van Beijsterveldt, R. C. M. Burgers, T. B. Vree, and E. van der Kleyn. J. Chromatogr. 415:419–422 (1987).
H. Weber, H. Spahn, E. Mutschler, and W. Möhrke. J. Chromatogr. 307:145–153 (1984).
H. Spahn and W. Henke. Naunyn-Schmiedeberg Arch. Pharmacol. 329(Suppl.):R11 (1985).
Ciba-Geigy. Final Report of Ciba-Geigy on CGP 11.130, Study No. D 111 A2/ ND 002, Wehr, FRG, 1981.
H. Spahn. Drug Res. (in preparation).
H. Spahn, H. Weber, E. Mutschler, and W. Mörke. J. Chromatogr. 310:167–178 (1984).
W. Bähr and H. Theobald. Organische Stereochemie: Begriffe und Definitionen, Springer, Berlin, Heidelberg, New York, 1973.
J. Hermansson and C. von Bahr. J. Chromatogr. 221:109–117 (1980).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Spahn, H., Krauβ, D. & Mutschler, E. Enantiospecific High-Performance Liquid Chromatographic (HPLC) Determination of Baclofen and Its Fluoro Analogue in Biological Material. Pharm Res 5, 107–112 (1988). https://doi.org/10.1023/A:1015992218497
Issue Date:
DOI: https://doi.org/10.1023/A:1015992218497