Abstract
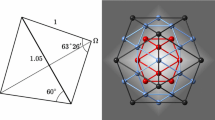
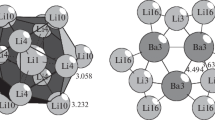
Mackay introduced two important crystallographic concepts in a short paper published 40 years ago. One is the icosahedral shell structure (iss) consisting of concentric icosahedra displaying fivefold rotational symmetry. The number of atoms contained within these icosahedral shells and subshells agrees well with the magic numbers in rare gas clusters, (C60) N molecules, and some metal clusters determined by mass spectroscopy or simulated on energy considerations. The cluster of 55 atoms within the second icosahedral shell occurs frequently and has been called Mackay icosahedron, or simply MI, which occurs not only in various clusters, but also in intermetallic compounds and quasicrystals. The second concept is the hierarchic icosahedral structures caused by the presence of a stacking fault in the fcc packing of the successive triangular faces in the iss. For instance, a fault occurs after the ABC layers resulting an ABCB packing. This is, in fact, a hierarchic icosahedral structure of a core icosahedron connected to 12 outer icosahedra by vertex sharing, or an icosahedron of icosahedra (double MI. Contrary to Mackay's iss, a faulted hierarchic icosahedral shell is, in fact, a twinlike face capping of the underlying triangles; it is, therefore, called an anti-Mackay cluster. The hierarchic icosahedral structure in an Al-Mn-Pd icosahedral quasicrystal has a core of body-centered cube rather than an icosahedron and, therefore, is called a pseudo-Mackay cluster. The hierarchic icosahedral structures have been studied separately in the past in the fields of clusters, nanoparticles, intermetallic compounds, and quasicrystals, but the underlying geometry should be the same. In the following a unified geometrical analysis is presented.
Similar content being viewed by others
REFERENCES
Mackay, A. L. Acta Crystallogr. 1962, 15, 916.
Pauling, L. J. Amer. Chem. Soc. 1947, 69, 542.
Frank, F. C. Proc. Roy. Soc. London 1952, A215, 43.
Ino, S. J. Phys. Soc. Jpn. 1966, 21, 346.
Allpress, J. G.; Sanders, J. V. Surf. Sci. 1967, 7, 1.
Volkov, V. V.; Van Tendeloo, G.; Tsirkov, G. A.; Cherkashina, N. V.; Vargaftik, M. N.; Moiseev, I. I.; Novotortsev, V. M.; Kvit, A. V.; Chuvilin, A. L. J. Cryst. Growth 1996, 163, 377.
Hofmeister, H. Cryst. Res. Technol. 1998, 33, 3.
Mackay, A. Nature (London) 1998, 391, 324.
Hubert, H.; Devouard, B.; Garvie, L. A. J.; O'Keeffe, M.; Buseck, P. R.; Petuskey, W. T.; McMillan, P. F. Nature (London) 1998, 391, 376.
Hoare, M. R.; Pal, P. J. Cryst. Growth 1972, 17, 77.
Hoare, M. R. Advan. Chem. Phys. 1979, 40, 49.
Farges, J.; Raoult, B.; Torcet, G. J. Chem. Phys. 1973, 59, 3454.
Echt, O.; Sattler, K.; Recknagel, E. Phys. Rev. Lett. 1981, 47, 1121.
Martin, T. P. Phys. Rep. 1996, 273, 199.
Martin, T. P.; Bergmann, T.; Göhlich, H.; Lange, T. Chem. Phys. Lett. 1991, 176, 343.
Martin, T. P.; Näher, U.; Bergmann, T.; Göhlich, H.; Lange, T. Chem. Phys. Lett. 1991, 183, 119.
Northby, J. A. J. Chem. Phys. 1987, 87, 6166.
Kroto, H. W.; Heath, J. R.; O'Brien, S. C.; Curl, R. F.; Smalley, R. E. Nature (London) 1985, 318, 162.
Martin, T. P.; Näher, U.; Schaber, H.; Zimmermann, U. Phys. Rev. Lett. 1993, 71, 3079.
Mackay, A. L. Izv. Jugosl. Centr. Krist. (Zagreb) 1975, 10, 15.
Penrose, R. Bull. Inst. Math. Appl. 1974, 10, 266.
Hargittai, I. Chem. Intell. 1997, 3, 25; Hargittai, I.; Hargittai, M. In Our Own Image, Personal Symmetry in Discovery; Kluwer/Academic: New York, 2000; p. 152.
Mackay, A. L. Physica 1982, 114A, 609.
Shechtman, D.; Blech, I.; Gratias, D.; Cahn, J. W. Phys. Rev. Lett. 1984, 53, 1951.
Elser, V.; Henley, C. L. Phys. Rev. Lett. 1985, 55, 2883.
Guyot, P.; Audier, M. Phil. Mag. B 1985, 52, L15.
Cooper, M.; Robinson, K. Acta Crystallogr. 1966, 20, 614.
Henley, C. L. Comments Condens. Matter Phys. 1987, 13, 59.
Sung, M.-W.; Kawai, R.; Weare, J. H. Phys. Rev. Lett. 1994, 73, 3552.
Yang, Q. B. Phil. Mag. B 1988, 58, 47.
Sugiyama, K.; Kaji, N.; Hiraga, K. Acta Crystallogr. 1998, C54, 445.
Hoare, M. Ann. N.Y. Acad. Sci. 1976, 279, 186.
Farges, J.; de Feraudy, M. F.; Raoult, B.; Torchet, G. J. Chem. Phys. 1986, 84, 3491.
Doye, J. P. K.; Wales, D. J.; Berry, R. S. J. Chem. Phys. 1995, 103, 4234.
Kreiner, G.; Franzen, H. F. J. Alloys Comp. 1995, 221, 15.
Hoard, I. L.; Sullenger, D. B.; Kennard, C. H. L.; Hughes, R. E. J. Solid State Chem. 1970, 1, 268.
Higashi, I.; Kobayashi, K.; Tanaka, T.; Ishizawa, W. J. Solid State Chem. 1997, 133, 16.
Cenzual, K.; Chabot, B.; Parthé, E. Acta Crystallogr. 1985, 41, 313.
Tamura, N. Phil. Mag. A 1997, 76, 337.
Samson, S. Acta Chem. Scand. 1949, 3, 809.
Samson, S. Acta Chem. Scand. 1949, 3, 835.
Bergman, G.; Waugh, J. L. T.; Pauling, L. Nature (London) 1952, 169, 1057.
Bergman, G.; Waugh, J. L. T.; Pauling, L. Acta Crystallogr. 1957, 10, 254.
Pauling, L. Phys. Rev. Lett. 1987, 58, 365.
Pauling, L. Amer. Scientist 1955, 43, 285.
Sun, W.; Lincoln, F. J.; Sugiyama, K.; Hiraga, K. Mater. Sci. Eng. 2000, 294–296, 327.
Cherkashin, E. E.; Kripyakevich, P. I.; Oleksiv, G. I. Sov. Phys.-Crystallogr. 1964, 8, 681.
Audier, M.; Pannetier, J.; LeBlanc, M.; Janot, C.; Lang, J.-M.; Dubost, B. Physica B 1988, 153, 136.
Boerdijk, A. H. Philips Res. Rept. 1952, 7, 303.
Frank, F. C.; Kasper, J. S. Acta Crystallogr. 1958, 11, 184; Acta Crystallogr. 1959, 12, 483.
Shoemaker, P. D.; Shoemaker, C. B. Acta Crystallogr. B, 1986, 42, 3.
Shoemaker, P. D.; Shoemaker, C. B. Mater. Sci. Forum 1987, 22–24, 67.
Samson, S. In Structural Chemistry and Molecular Biology; Rich, A.; Davidson, N., eds.; Freeman: San Fransisco, CA, 1968; p. 687.
Samson, S. Mater. Sci. Forum 1987, 22–24, 83.
Pauling, L. The Nature of the Chemical Bond and Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry, 3rd edn.; Cornell University Press: Ithaca, New York, 1963.
Mackay, A. L. Nature (London) 1985, 315, 636.
Ramanchandrarao, P.; Sastry, G. V. S. Pramana 1985, 25, L225.
Henley, C. L.; Elser, V. Phil. Mag. B 1986, 53, L59.
Audier, M.; Sainfort, P.; Dubost, B. Phil. Mag. B 1986, 54, L105.
Tillard-Chabonnel; Belin, C. J. Solid State Chem. 1991, 90, 270.
Tillard-Chabonnel; Chahine, A. Belin, C. Mater. Res. Bull. 1993, 28, 1285.
Tillard-Chabonnel; Belin, C. Mater. Res. Bull. 1992, 27, 1277.
Boudard, M.; Boissieu, M. D., Janot, C.; Heger, G.; Beeli, C.; Nissen, H.-U.; Vincent, H.; Ibberson, R.; Audier, M.; Dubois, J. M. J. Phys. Condens. Matter 1992, 4, 10149.
Janot, C.; de Boissieu, M. Phys. Rev. Lett. 1994, 72, 1674.
Mahne, S.; Steurer, W. Z. Kristallogr. 1996, 211, 17.
Edler, F. J.; Gramlich, V.; Steurer, W. J. Alloys Comp. 1998, 269, 7.
Hiraga, K.; Suiyama, K.; Ohsuna, T. Phil. Mag. A 1998, 78, 1051.
Tsai, A. P.; Guo, J. Q.; Abe, E., Takakura, H.; Sato, T. J. Nature (London) 2000, 408, 537.
Takakura, H.; Guo, J.; Tsai, A. P. Phil. Mag. Lett. 2001, 81, 411.
Palenzona, A. J. Less-Common Met. 1971, 25, 367.
Bruzzone, G. Gazz. Chim. Italy 1972, 102, 234.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kuo, K.H. Mackay, Anti-Mackay, Double-Mackay, Pseudo-Mackay, and Related Icosahedral Shell Clusters. Structural Chemistry 13, 221–230 (2002). https://doi.org/10.1023/A:1015847520094
Issue Date:
DOI: https://doi.org/10.1023/A:1015847520094