Abstract
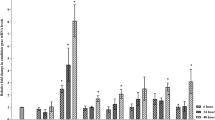
The antifungal variant from Taxus callus has been screened. The accumulation of biomass and paclitaxel of resistant and wild-type cells on modified MS medium or treated by fungal-elicitor was compared. The results showed that the production of biomass and paclitaxel of resistant cells was approximate as that of the wild-type cells on normal modified MS medium, but it was 70-fold higher when stimulated by fungal-elicitor. And there were evident difference on the activity of peroxidase (POD), phenylalanine ammonialyase (PAL) and the content of superoxide anion (O2 −) between the antifungal variant and wild-type cells. The results implied that the intensity and velocity of the occurrence of secondary metabolism of antifungal variant were higher than those of the wild-type cells and the selection of resistant variant will be a good new path to improve paclitaxel production.
Similar content being viewed by others

References
Baker CJ & Orlandi EW (1995) Active oxygen in plant pathogenesis. Annu. Rev. Phytopathol. 33: 299–321
Byun SY, Tyu YW, Kim C & Pederson H (1992) Elicitation of sanguinarine production in two phase cultures of Eschscholtzia californica. J. Ferm. Bioeng. 73: 380–385
Chai HB & Doke N (1987) Activation of potential of potato leaf tissue to react hypersensitively to Phyhtophthora infestans by cytospore germination fluid and the enhancement of this potential by calcium ions. Physiol. Mol. Plant Pathol. 30: 27–37
Cragg GM & Snader KM (1991) Paclitaxel the supply issue. Cancer Cells Mon. Rev. 3: 233–235
Discosmo F & Misawa M (1985) Eliciting secondary metabolism in plant cell cultures. Trends Biotechnol. 3: 318
Dixon RA & Harrison MJ (1990) Activition structure and organization of genes involved in microbial defense plants. Adv. In Gene. 28: 165–171
Doke N (1983) Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytopathora infestans and to the hyphal cell wall components. Physiol. Plant Pathol. 23: 345–357
Eliert U (1987) Elicitation: methodology and aspects of application. Cell Culture and Somalic Cell Genetics of Plants, Vol. 4 Academic Press, New York (pp 153)
Hammerschmidt R, Nuckles EM & Kuc J (1982) Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrchum lagenarium. Physiol. Plant. Pathol. 20: 73–82
Hortobagyi GN, Holmes FA, Heriault RL & Bozdar AD (1994) Use of Paclitaxel (Paclipaclitaxel) in Breast Cancer. Oncology 51 (suppl 1): 29–32
Jiao RS, Li ZP & Li ZX (1994) Cell Engineering. Chemical Industry Press, Beijing
Kim DJ & Chang HN (1990) Increased shikonin production in Lithospermun crythrorhizon suspension cultures with in situ extract and fungal cell treatment (elicitor). Biotechnol. Lett. 12: 443–449
Kong XS, Zhang MX, Du AL & Xu XZ (1998) Comparison and improvement of the measurement method of soluble sugar in plant tissue. Journal of Central China Agricultural University 26: 49–52
Lu MB, Su X & Mei XG (1998) Effect of fungal-elicitor on the cell suspensions of Taxus chinesis. J. Huazhong Univ. of Sci. & Tech. 26: 107–110
Mansfield JW, Hargreaves JA & Boyle FC (1974) Phytoalexin production by live cells in broad bean leaves infected with Botrytris cinerea. Nature 252: 316–319
Mei XG, Lu MB, Yu LJ & Hu DW (1996) Kinetics of Paclitaxel biosynthesis in bioreactors. Med. Chem. Res. 6: 256–241
Murashige T & Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol. Plant. 15; 473–497
Ning W & Chao RQ (1994) Effects of fungal elicitor on shikonin derivatives formation in Onsosma paniculatum cell cultures, Plant Physiol. Comm. 30: 348–350
Patterson BD, Mackae EA & Ferguson JB (1984) Estimation of hydrogen peroxide in plant extracts using Titanium (IV), Annal. Biochem. 139: 487–492
Rowinsky EK & Donehower RC (1993) The clinical pharmacology of Paclitaxel (Paclitaxel). Semin Oncol. 20(4 suppl 3): 16–25
Sequeira LM (1983) Mechanisms of induced resistance in plants. Annu. Rev. Microbiol. 37: 51–57
Shuler ML (1994) Bioreactor engineering as an enabling technology to tap biodiversity: The case of paclitaxel. Ann. N.Y. Acad. Sci. 745: 455
Stewart RC & Bewley JD (1980) Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 65: 245–248
Stull D P (1992) Current paclitaxel production from yew bark and future production strategies. Paper presented at the Second National Cancer Institute workshop on paclitaxel and Taxus. Sept. 23–24, Alexandria, VA
Wickremesinhe ERM & Arteca RN (1994) Taxus cell suspension cultures: Optimizing growth and production of paclitaxel. J. Plant Physiol. 144: 183–188
Yu SW & Tang ZC (1998) Plant Physiology and Plant Molecular Biology. Science Press, Beijing
Zucker M(1965) Induction of phenylalanine deaminase by light and its relation to chlorogenic acid synthesis in potato tuber tissues. Plant Physiol. 40: 779–784
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Su, X., Mei, X. & Gong, W. Study of paclitaxel prodution from an antifungal variant of Taxus callus. Plant Cell, Tissue and Organ Culture 68, 215–223 (2002). https://doi.org/10.1023/A:1013942719483
Issue Date:
DOI: https://doi.org/10.1023/A:1013942719483