Abstract
Background: Focussing on regional cerebral hypoperfusion during hemodynamically stable, but borderline hypotensive, sustained ventricular tachycardia (VT) experimental studies show (1) a reduction of cerebral blood flow (CBF) during tachyarrhythmias in contrast to the concept of CBF autoregulation, (2) a mediation of hypoperfusion by neuronal and humoral mechanisms, and (3) an involvment of microcirculation due to an ischemic stress response of the cerebral tissue. The clinical relevance of these observations remains still unclear.
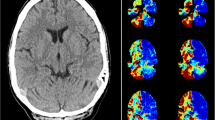
Case reports: Two patients with coronary artery disease, left ventricular dysfunction and sustained monomorphic VT underwent electrophysiological study. VT was induced and the tracer 99mTc-HMPAO was injected after 3 minutes of ongoing VT. Regional CBF during this life threatening arrhythmia was determined with brain SPECT. A scanning protocol was performed after termination of VT. The measurements were repeated at baseline during normofrequent sinus rhythm (SR) one week later. CBF during SR was significantly reduced in the temporal lobe in comparison to the conditions during stable VT, particularly in the left hippocampus.
Conclusion: The reduction of hippocampal CBF due to cerebrovascular vasoconstriction and neuronal reflex mechanism previously observed in experiments during stable, sustained VT can be confirmed in a clinical scenario by high resolution 99mTc-HMPAO brain SPECT. This supports the hypothesis that repetitive stable VT can play a role in the pathophysiology of cerebrovascular insufficiency. Further clinical studies are needed to analyze the impact of tachyarrhythmias on cognitive and mnemic function.
Similar content being viewed by others
References
Harper AM. Autoregulation of cerebral blood flow: influence of the arterial blood pressure on the blood flow through the cerebral cortex. J Neurol Neurosurg Psychiatry 1966;29:389-403.
Heiss WD, Traupe H. Comparison between hydrogen clearance and microsphere technique for rCBF measurement. Stroke 1981;12:161-167.
Lacombe P, Meric P, Seylaz J. Validity of cerebral blood flow measurements obtained with quantitative techniques. Brain Res Rev 1980;2:105-169.
Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovascular and Brain Metabolism Reviews 1990;2:161-192.
Grubb BP, Gerard C, Roush K. Cerebral vasoconstriction during head upright tilt induced vasovagal syncope: A paradoxic and unexpected response. Circulation 1991;84:1157-1164.
Grubb BP, Durzinsky D, Brewster P, Gbur C, Collins B. Sudden cerebral vasoconstriction during induced polymorphic ventricular tachycardia and fibrilation: Further observations of a paradoxic response. PACE 1997;20:1667-1672.
Blomquist P, Wieloch T. Ischemic brain damage in rats following cardiac arrest using a long-term recovery model. J Cereb Blood Flow Metabol 1985;5, 420-431.
Hagendorff A, Dettmers C, Danos P, Pizzulli L, Omran H, Manz M, Hartmann H, Lüderitz B. Myocardial and cerebral hemodynamics during tachyarrhythmiainduced hypotension in the rat. Circulation 1994;90:400-410.
Hagendorff A, Dettmers C, Block A, Pizzulli L, Omran H, Hartmann A, Manz M, Lüderitz B. Reduction of cerebral blood flow with induced tachycardia in rats and in patients with coronary artery disease and premature ventricular contractions. Eur Heart J 1994;15:1477-1481.
Hagendorff A, Dettmers C, Danos P, Hümmelgen M, Vahlhaus C, Martin M, Heusch G, Lüderitz B. Cerebral vasoconstriction during sustained ventricular tachycardia induces an ischemic stress response of brain tissue in rats. J Mol Cell Cardiol 1998;30:2081-2094.
Kastrup A, Hagendorff A, Dettmers C, Lüderitz B, Hartmann A. Hemodynamic sequelae of ventricular tachyarrhythmias on cerebral and renal blood flow. Neurol Res 1998;20:549-554.
Kobari M, Fukuuchi Y, Tomita M, Tanahashi N, Shinohara T, Yamawaki T, Ohta H, Takeda H. Cerebral microcirculatory changes during and following transient ventricular tachycardia in cats. J Neurol Sci 1992;111:153-157.
Ginsberg MD, Busto R. Progress review: rodent models of cerebral ischemia. Stroke 1989;20:1627-1642.
Garcia JH, Lassen NA, Weiller C, Sperling B, Nagawara J. Ischemic stroke and incomplete infarction. Stroke 1996;27:761-765.
Lassen NA. Incomplete cerebral infarction: Focal incomplete ischemic tissue necrosis not leading to emolission. Stroke 1982;13:522-523.
Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol 1994;36:557-565.
Kirino T, Tamura A, Sano K. Delayed neuronal death in the rat hippocampus following transient forebrain ischemia. Acta Neuropathol 1984;64:139-147.
Morioka T, Kalehua AN, Streit WJ. The microglial reaction in the rat dorsal hippocampus following transient forebrain ischemia. J Cereb Blood Flow Metab 1991;11:966-973.
Nakano S, Kato H, Kogure K. Neuronal damage in the rat hippocampus in a new model of repeated reversible transient cerebral ischemia. Brain Res 1989;490:178-180.
Ineoue T, Kato H, Araki T, Kogure K. Emphasized selective vulnerability after repeated nonlethal cerebral ischemic insults in rats. Stroke 1992;23:739-745.
Englund E, Brun A. White matter changes in dementia of Alzheimer's type: the difference in vulnerability between cell components. Histopathology 1990;16:433-439.
Englund E, Brun A, Svennerholm L. White matter gangliosides in incomplete infarction. Dementia 1990;1:286-291.
Herholz K, Heindel W, Rackl A, Neubauer I, Steinbrich W, Pietrzyk U, Erasmil-Körber H, Hess WD. Regional cerebral blood flow in patients with leukoaraiosis and atherosclerotic carotid artery disease. Arch Neuro 1990;47:392-396.
Davis HP, Baranowski JR, Pulsinelli WA, Volpe BT. Retention of reference memory following ischemic hippocampal damage. Physiol Behav 1987;39:783-786.
de la Torre JC, Fortin T, Park GAS, Butler KS, Kozlowski P, Pappas BA, de Socarraz H, Saunders JK, Richard MT. Chronic cerebrovascular insuffi ciency induces dementia-like deficits in aged rats. Brain Res 1992;582:186-195.
Kiyota Y, Miyamoto M, Nagaoka A, Nagawa Y. Cerebral embolization leads to memory impairment of several learning tasks in rats. Pharmakol Biochem Behav 1986;24:687-692.
Kuribayashi Y, Naritomi H, Nuluzuma S. Multiple cerebral infarcts and white matter changes caused by chronic low flow perfusion in rats. J Neurol 1990;237:155-158.
Sekhon LHS, Morgan MK, Spence I, Weber NC. Chronic cerebral hypoperfusion and impaired neuronal function in rats. Stroke 1994;25:1022-1027.
Ell PJ, Cullum ID, Costa DC, Jarritt PH, Hocknell JML, Lui D, Jewkes RJ, Steiner TJ, Nowotnik DP, Pickett RD, Neirinckx RD. A new regional cerebral blood flow mapping with 99mTc-labelled compound. Lancet 1985;II:50-51.
Lassen NA, Andersen AR, Friberg L, Paulson OB. The retention of [99mTc]-d,l-HM-PAO in the human brain after intracarotid bolus injection: A kinetic analysis. J Cereb Blood Flow Metab 1988;8(suppl. 1) S13-S22.
Nowotnik DP, Canning, LR, Cumming SA, Harrison RC, Highley B, Nechvatal G, Pickett RD, Piper IM, Bayne VJ, Forster AM, Weisner PS, Neirinckx RD. Development of a 99mTc-labelled radiopharmaceutical for cerebral blood flow imaging. Nucl Med Commun 1985;6:499-506.
Tyrrell DA. Amersham Report N 109/28. European Phase I and Phase II Clinical Trials. Amersham 1985.
Genna S, Smith AP. The development of ASPECT, an annular single crystal brain camera for high efficiency SPECT. IEEE Trans Nucl Sci NS 1988;35:654-658.
Holman BL, Carvalho PA, Zimmerman RE, et al. Brain perfusion SPECT using an annular single crystal camera: Initial clinical experience. J Nucl Med 1990;31:1456-1461.
Lavy S, Stern S, Melamed E, Cooper G, Keren A, Levy P. Effect of chronic atrial fibrillation on regional cerebral blood flow. Stroke 1980;11:35-38.
Petersen P, Kastrup J, Videbaek R, Boysen G. Cerebral blood flow before and after cardioversion of atrial fibrillation. J Cereb Blood Flow Metab 1989;9:422-425.
Brun A. Pathology and pathophysiology of cerebrovascular dementia: pure subgroups of obstructive and hypoperfusive etiology. Dementia 1994;4:145-147.
Dobkin BH. Orthostatic hypotension as a risk factor for symptomatic occlusive cerebrovascular disease. Neurology 1989;39:30-34.
Hagendorff A, Pizzulli L, Dettmers C, Block A, Omran H, Hartmann A, Manz M, Lüderitz B. Transient focal cerebral ischemia during hypotension due to pacemaker syndrom. Z Kardiol 1994;83:908-911.
Hagendorff A, Dettmers C, Jung W, Hümmelgen M, Kölsch C, Hartmann A, Lüderitz B, Pfeiffer D. Optimizing cerebral blood flow by pacemaker therapy-a possible issue to prevent of cerebrovascular diseases? Dtsch med Wschr 2000;125:286-289.
Hagendorff A, Kölsch C, Dettmers C, Hartmann A, Pfeiffer D, Lüderitz B. Is it possible to introduce changes of cerebral blood flow by changes of cardiac output-measurements of cerebral blood flow in controls during volume overload and in pacemaker patients by changing the pacing rate. Z Kardiol 2001;90:35-42.
Dettmers C, Hagendorff A, Lüderitz B, Hartmann A. Repetitive hypotensive episodes-their contribution to vascular cognitive impairment. Nervenarzt 1997;68:625-632.
Rosen SD, Paulescu E, Nihoyannapoulos P, Tousoulis D, Frackowiak RSJ, Frith CD, Jones T, Camici PG. Silent ischemia as a central problem: regional brain activation compared in silent and painful myocardial ischemia. Ann Intern Med 1996;124:939-949.
Naritomi H, Sasaki M, Kanashiro M, Sawada T, Kadota E, Hashimoto S. Experimental approaches to vascular dementia. Rec Adv Cardiovasc Dis 1990;11:61-68
Volpe BT, Waczek B, Davis HP. Modified T-maze training demonstrates dissociated memory loss in rats with ischemic hippocampal injury. Behav Brain Res 1988;27:259-268.
Volpe BT, Davis HP, Colombo PJ. Preoperative training modifies radial maze performance in rats with ischemic hippocampal injury. Stroke 1989;20:1700-1706.
Breteler MMB, van Amerongen NM, van Swieten JC, Claus JJ, Grobbee DE, vam Gijn J, Hofman A, van Harskamp F. Cognitive correlates of ventricular enlargement and cerebral white matter lesions on magnetic resonance imaging. Stroke 25;1994:1109-1115.
O'Brien MD: How does cerebrovascular disease cause dementia? Dementia 1994;5:133-136.
Ylikowski R, Ylikowski A, Erkinjuntti T, Sulkava R, Raininko R, Tilvis R. White matter changes in healthy elderly persons correlate with attenuation and speed of mental processing. Arch Neurol 1993;50:818-824.
Emerson TR, Milne JR, Gardner AJ. Cardiogenic dementia-a myth? Lancet 1981;2:743-744.
Mecklinger A, von Cramon Y, Matthes-von Cramon G. Event-related potential evidence for a specific recognition memory deficit in adult survivors of cerebral hypoxia. Brain 1998;121:1919-1935.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hagendorff, A., Klemm, E., Bangard, M. et al. Case Report: Regional Cerebral Hypoperfusion Induced by Ventricular Tachycardia – Short-term Hippocampal Hypoperfusion and its Potential Relationship to Selective Neuronal Damage. J Interv Card Electrophysiol 5, 435–441 (2001). https://doi.org/10.1023/A:1013202213276
Issue Date:
DOI: https://doi.org/10.1023/A:1013202213276