Abstract
The activity of ATP, ubiquitin (Ub)‐dependent proteases partially purified from skeletal muscle (psoas) from alloxan diabetic rabbits was determined at different periods of insulin deficiency. Two days after alloxan injection, no change was observed in the activity of ATP, Ub‐dependent proteases, but this activity increased 3 and 5 days after diabetes induction, attaining 181% of control values on the 5th day. However, after this early rise, the activity of muscle ATP, Ub‐dependent proteases decreased, returning to values that did not differ significantly from controls 7 and 10 days after alloxan injection. After 15 days, the activity of these proteases was 57% lower than in muscle from control rabbits. Both the initial increase and the subsequent fall in the activity of the enzymes were prevented by insulin treatment of alloxan diabetic rabbits. The data suggest that Ub‐proteasome‐dependent proteolysis have an important role in the control of muscle protein degradation and may be regulated by insulin.
Similar content being viewed by others
References
Bloomart EF, Luiken JJ, Meijer AJ: Autophagic proteolysis: Control and specificity. Histochem J 29: 365–385, 1997
Knop M, Schiffer HH, Rupp S, Wolf DH: Vacuolar/lysosomal proteolysis: Proteases, substrates, mechanisms. Curr Opin Cell Biol: 990–996, 1993
Furuno K, Goldberg AL: The activation of protein degradation in muscle by Ca2+ or muscle injury does not involve a lysosomal mechanism. Biochem J 237: 859–864, 1986
Huang J, Forsberg NE: Role of calpain in skeletal-muscle protein degradation. Proc Natl Acad Sci USA 95: 12100–12105, 1998
Navegantes LCC, Resano NMZ, Migliorini RH, Kettelhut IC: Effect of guanethidine-induced adrenergic blockade on the different proteolytic systems in rat skeletal muscle. Am J Physiol Endocrinol Metab 277: E883–E889, 1999
Fagan JM, Waxman L: The ATP-independent pathway in red blood cells that degrades oxidant-damaged hemoglobin. J Biol Chem 267: 23015–23022, 1992
Etlinger JD, Goldberg AL: A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc Natl Acad Sci USA 74: 54–58, 1977
Wilk S, Orlowski M: Cation-sensitive neutral endopeptidase: Isolation and specificity of the bovine pituitary enzyme. J Neurochem 35: 1172–1182, 1980
Driscoll J, Goldberg AL: The proteasome (multicatalytic protease) is a component of the 1500-kDa proteolytic complex which degrades ubiquitin-conjugated proteins. J Biol Chem 265: 4789–4792, 1990
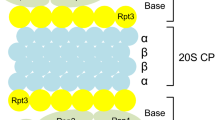
Voges D, Zwickl P, Baumeister W: The 26S proteasome: A molecular machine designed for controlled proteolysis. Annu Rev Biochem 68: 1015–1068, 1999
Attaix D, Combaret L, Pouch MN, Taillandier D: Regulation of proteolysis. Curr Opin Clin Nutr Metab Care 4: 45–49, 2001
Ding X, Price SR, Bailey JL, Mitch WE: Cellular mechanisms controlling protein degradation in catabolic states. Miner Electrolyte Metab 23: 194–197, 1997
Dardevet D, Sornet C, Taillander D, Savary I, Attaix D, Grizard J: Sensitivity and protein turnover response to glucocorticoids are different in skeletal muscle from adult and old rats. Lack of regulation of the ubiquitin-proteasome proteolytic pathway in aging. J Clin Invest 96: 2113–2119, 1995
Rannels DE, Kao R, Morgan HE: Effect of insulin on protein turnover in heart muscle. J Biol Chem 250: 1694–1701, 1975
Mortimore GE, Ward WF, Schworer CM: Lysosomal processing of intracellular protein in rat liver and its general regulation by amino acids and insulin. In: H.L. Segal, D.J. Doyle (eds). Protein Turnover and Lysosome Function. Academic Press, New York, 1978, pp 67–68
Li JB, Wassner SJ: Effects of food deprivation and refeeding on total protein and actomyosin degradation. Am J Physiol Endocrinol Metab 246: E32–E37, 1984
Smith DM, Sugden PH: Contrasting response of protein degradation to starvation and insulin as measured by release of N-methylhistidine or phenylalanine from the perfused heart. Biochem J 237: 391–395, 1986
Price SR, Bailey JL, Wang X, Jurkovitz C, England BK, Ding X, Phillips LS, Mitch WE: Muscle wasting in insulinopenic rats results from activation of the ATP-dependent, ubiquitin-proteasome proteolytic pathway by a mechanism including gene transcription. J Clin Invest 98: 1703–1708, 1996
Merforth S, Osmers A, Dahlmann B: Alterations of proteasome activities in skeletal muscle tissue of diabetic rats. Mol Biol Rep 26: 83–87, 1999
Lecker SH, Solomon V, Price SR, Kwon YT, Mitch WE, Goldberg AL: Ubiquitin conjugation by the N-end rule pathway and mRNA for its components increase in muscles of diabetic rats. J Clin Invest 104: 1411–1420, 1999
Mitch WE, Bailey JL, Wang X, Jurkovitz C, Newby D, Price SR: Evaluation of signals activating ubiquitin-proteasome proteolysis in a model of muscle wasting. Am J Physiol 275: C1132–C1138, 1999
Bailey JL, Wang X, Price SR: The balance between glucocorticoids and insulin regulates muscle proteolysis via the ubiquitin-proteasome pathway. Miner Electrolyte Metab 25: 220–223, 1999
Hamel FG, Bennet RG, Duckworth WC: Regulation of multicatalytic enzyme activity by insulin and the insulin-degrading enzyme. Endocrinology 139: 4061–4066, 1998
Duckworth WC, Bennett RG, Hamel FG: Insulin acts intracellularly on proteasomes through insulin-degrading enzyme. Biochem Biophys Res Commun 244: 390–394, 1998
Pepato MT, Migliorini RH, Goldberg AL, Kettelhut IC: Role of different proteolytic pathways in degradation of muscle protein from streptozotocin-diabetic rats. Am J Physiol Endocrinol Metab 271: E340–E347, 1996
Fagan JM, Waxman L, Goldberg AL: Skeletal muscle and liver contain a soluble ATP + ubiquitin-dependent proteolytic system. Biochem J 243: 335–343, 1987
Read SM, Northcote DH: Minimization of variation in the response to different proteins of the Coomassie Blue G dye-binding assay for protein. Anal Biochem 116: 53–64, 1981
Waalkes TP, Udenfriend S: A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med 50: 733–736, 1957
Driscoll J, Goldberg AL: The skeletal muscle proteasome can degrade proteins in an ATP-dependent process that is independent of ubiquitin. Proc Natl Acad Sci USA 74: 54–58, 1989
Saito Y, Tsubuki S, Ito H, Kawashima S: The structure-function relationship between peptide aldehyde derivatives on initiation of neurite outgrowth in PC12h cells. Neurosci Lett 120: 1–4, 1990
Kadish, AH, Little RL, Sternberg JC: A new and rapid method for the determination of glucose by measurement of rate of oxygen consumption. Clin Chem 14: 116–131, 1968
Dole VP, Meinertz H: Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem 235: 2595–2599, 1960
Liu Z, Miers WR, Wei L, Barrett EJ: The ubiquitin-proteasome proteolytic pathway in heart vs. skeletal muscle: Effects of acute diabetes. Biochem Biophys Res Commun 276: 1255–1260, 2000
Wing SS, Goldberg AL: Glucocorticoids activate the ATP-ubiquitindependent proteolytic system in skeletal muscle during fasting. Am J Physiol Endocrinol Metab 264: E668–E676, 1993
Grizard J, Dardevet D, Balage M, Larbaud D, Sinaud S, Savary I, Grzelkowska K, Rochon C, Tauveron I, Obled C: Insulin action on skeletal muscle protein metabolism during catabolic states. Reprod Nutr Dev 39: 61–74, 1999
Miers WR, Barrett EJ: The role of insulin and other hormones in the regulation of amino acid and protein metabolism in humans. J Basic Clin Physiol Pharmacol 9: 235–253, 1998
Medina R, Wing SS, Haas A, Goldberg AL: Activation of the ubiquitin ATP-dependent proteolytic system in skeletal muscle during fasting and denervation atrophy. Biochem Biomed Acta 50: 347–356, 1991
Solomon V, Baracos V, Sarraf P, Goldberg AL: Rates of ubiquitin conjugation increase when muscles atrophy, largely through activation of the N-end rule pathway. Proc Natl Acad Sci USA 95: 12602–12607, 1998
Wing SS, Banville D: 14-kDa ubiquitin-conjugating enzyme: Structure of the rat gene and regulation upon fasting and by insulin. Am J Physiol 267: E39–E48, 1994
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galban, V.D., Evangelista, E.A., Migliorini, R.H. et al. Role of ubiquitin‐proteasome‐dependent proteolytic process in degradation of muscle protein from diabetic rabbits. Mol Cell Biochem 225, 35–41 (2001). https://doi.org/10.1023/A:1012260605910
Issue Date:
DOI: https://doi.org/10.1023/A:1012260605910