Abstract
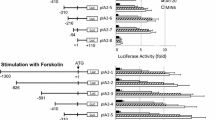
A −1027 bp to +108 bp region of Na-K-ATPase α3 gene promoter has been searched for the presence of thyroid response elements (TRE). Computer analysis of this sequence using a consensus TRE sequence revealed the presence of four putative TRE rich regions referred to as regions I (−636 to −457 bp), II (−218 to −106 bp), III (−106 to −6 bp) and IV (−6 to +108 bp). Cotransfection of the luciferase linked full length construct as well as constructs progressively devoid of the TRE rich regions in Cos1 cells revealed that regions I and III are positively regulated by T 3 whereas there are some sequences in region II which can suppress the positive regulatory effect of region III but not of region I, TRE IV seems to have no functional role. EMSA of the three functional TRE rich regions (I, II and III) showed strong and specific interaction with thyroid hormone receptor (TR) cloned and expressed in baculovirus. The overall results suggest the regulation of Na-K-ATPase α3 gene by T 3 is complex involving several thyroidal regulatory elements.
Similar content being viewed by others
References
Crambert G, Hasler U, Beggah AT, Yu C, Modyanov NN & Berger H & Geering K (2000) J. Biol. Chem. 275: 1976–1986
Pestov NB, Adams G, Shakhparonov MI & Modyanov NN (1999) FEBS Lett. 456: 243–248
Shull GE, Greeb J & Lingrel JB (1986) Biochemistry 25: 8125–8132
Herrera VLM, Emanuel JR., Ruiz-Opazo N, Levenson R. & Nadal-Ginard B. (1987) J. Cell. Biol. 105: 1855–1865
Mercer RW, Schneider JW, Savitz A, Emmanuel JR, Benz EJ. & Levenson R (1986) Mol. Cell. Biol. 6: 3884–3890
Martin Vasallo P, Dackowski W, Emanuel JR. & Levenson R (1989) J. Biol. Chem. 264: 4613–4618
Watts AG., Watts GS, Emmanuel JR & Levenson R (1991) Neurobiology 88: 2725–2729
Orlowski J. & Lingrel JB. (1988) J. Biol. Chem. 263: 10436–10444
Chaudhury S, Bajpai M. & Bhattacharya S (1996) J. Mol. Neurosci. 7: 229–234
Bajpai M & Chaudhury S (1999) NeuroReport 10: 2325–2328
Kamitani T, Ikeda U, Muto S, Kawakami K, Nagano K., Tsuruya, He H, Chin S, Zhuaug K, Hartong R, Apriletti J, & Gick, G. (1996) Am. J. Physiol. 271: C1750–1756
Luo Z, Singh IS, Fugihara T & Erlichman J (1992) J. Biol. Chem. 267: 24738–24747
Crew MD & Spindler SR (1986) J. Biol. Chem. 261: 5018–5022
Norman MF, Lavin TN, Baxter JD & West BL (1989) J. Biol. Chem. 264: 12063–12073
Hollenberg AN, Monden T, Flynn TR, Boers ME, Cohen O & Wondisford FE (1995) Mol. Endocrinol. 9: 540–550
Izumo S & Mahadavi V (1988) Nature 334: 539–542
Breen JJ, Hickok NJ & Gurr JA (1997) Mol. Cell. Endocrinol. 5: 137–146
Day RN & Maurer RA (1989) Mol. Endocrinol. 3: 931–938
Giral M, Park EA, Gurney AL, Liu JS, Hakimi F & Hanson RW (1991) J. Biol. Chem. 266: 21991–21996
Martin I, Villeno JA, Giratt M, Iglesias R, Mampel T, Vinas O & Villarroya F. (1996) Mol. Cell. Biochem. 154: 107–111
Farsetti A, Desvergne B, Hallenbeck P, Robbins J & Nikodem VM. (1992) J. Biol. Chem. 267: 15784–15788
Zgombic M & Duester (1993) Ad. Exp. Med. Biol. 328: 571–580
Adam RA, Cox JJ, Van Kats JP & Burbach JP (1992) J. Biol. Chem. 267: 3771–3777
Suzuki S, Miyamoto T, Opsahl A, Sakurai A & DeGroot IJ (1994) Mol. Endocrinol. 8: 305–314
Farsetti A, Mitsuhashi T, Desvergne B, Robbins J, & Nikodem VM (1991) J. Biol. Chem. 266: 23226–23232
Desvergne B, Petty KJ & Nikodem VM (1991) J.Biol.Chem. 266: 1008–1013
Collingwood. TN, Urnov FD & Wolffe AP (1999) J. Mol. Endocrimol. 23: 255–275
Chin S, Apriletti J & Gick G (1998) Endocrinology 139: 3423–3431
Corthesy-Theulaz J, Merillat AM, Honeggar P & Rossier BC (1991) Am. J. Physiol. 261: C124–13129
Patricia AW, Crew MD & Spindler SR (1987) J. Biol. Chem. 262: 5653–5663
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bajpai, M., Mandal, S.K. & Chaudhury, S. Identification of thyroid regulatory elements in the Na-K-ATPase α3 gene promoter. Mol Biol Rep 28, 1–7 (2001). https://doi.org/10.1023/A:1011986418897
Issue Date:
DOI: https://doi.org/10.1023/A:1011986418897