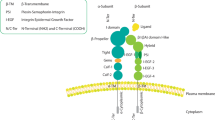
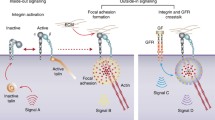
Abstract
This review explores the mechanistic basis of breast carcinoma progression by focusing on the contribution of integrins. Integrins are essential for progression not only for their ability to mediate physical interactions with extracellular matrices but also for their ability to regulate signaling pathways that control actin dynamics and cell movement, as well as for growth and survival. Our comments center on the α6 integrins (α6β1 and α6β4), which are receptors for the laminin family of basement membrane components. Numerous studies have implicated these integrins in breast cancer progression and have provided a rationale for studying the mechanistic basis of their contribution to aggressive disease. Recent work by our group and others on mechanisms of breast carcinoma invasion and survival that are influenced by the α6 integrins are discussed.
Similar content being viewed by others
REFERENCES
E. R. Fearon (1999). Cancer progression. Curr. Biol. 9:R873-875.
D. Hanahan and R. A. Weinberg (2000). The hallmarks of cancer. Cell 100:57-70.
E. Rodriguez-Boulan and W. J. Nelson (1989). Morphogenesis of the polarized epithelial cell phenotype. Science 245:718-725.
H. Colognato and P. Yurchenco (2000). Form and function: The laminin family of heterotrimers. Developmental Dynamics 218:213-234.
N. Boudreau and M. J. Bissell (1998). Extracellular matrix signaling: Integration of form and function in normal and malignant cells. Curr. Opin. Cell. Biol. 10:640-646.
M. J. Bissell, V. M. Weaver, S. A. Lelievre, F. Wang, O. W. Petersen, and K. L. Schmeichel (1999). Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res. 59:1757-1763s.
R. S. Cotran, V. Kumar, and T. Collins (1999). Robbins Pathological Basis of Disease. Saunders, Philadelphia.
T. Tani, A. Lumme, A. Linnala, E. Kivilaakso, T. Kiviluoto, R. E. Burgeson, L. Kangas, I. Leivo, and I. Virtanen (1997). Pancreatic carcinomas deposit laminin-5, preferably adhere to laminin-5, and migrate on the newly deposited basement membrane. Am. J. Pathol. 151:1289-1302.
H. Mizushima, H., Y. Miyagi, Y. Kikkawa, N. Yamanaka, H. Yasumitsu, K. Misugi, and K. Miyazaki (1996). Differential expression of laminin-5/ladsin subunits in human tissues and cancer cell lines and their induction by tumor promoter and growth factors. J. Biochem. (Tokyo). 120:1196-1202.
L. M. Shaw, I. Rabinovitz, H. H. Wang, A. Toker, and A. M. Mercurio (1997). Activation of phosphoinositide 3-OH kinase by the α6β4 integrin promotes carcinoma invasion. Cell 91:949-960.
R. E. Bachelder, M. J. Ribick, A. Marchetti, R. Falcioni, S. Soddu, K. R. Davis, and A. M. Mercurio (1999). p53 inhibits α6β4 integrin survival signaling by promoting the caspase 3-dependent cleavage of AKt/PKB. J. Cell. Biol. 147:1063-1072.
G. E. Plopper, S. Z. Domanico, V. Cirulli, W. B. Kiosses, and V. Quaranta (1998). Migration of breast epithelial cells on Laminin-5: Differential role of integrins in normal and transformed cell types. Breast Cancer Res. Treat. 51:57-69.
N. Koshikawa, G. Giannelli, V. Cirulli, K. Miyazaki, and V. Quaranta (2000). Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J. Cell. Biol. 148:615-624.
A. Mercurio (1995). Receptors for the laminins. Achieving specificity through cooperation. Trends in Cell biology 5:419-423.
L. M. Shaw (1999). Integrin function in breast carcinoma progression. J. Mammary Gland. Biol. Neoplasia 4:367-376.
K. Friedrichs, P. Ruiz, F. Franke, I. Gille, H. J. Terpe, and B. A. Imhof (1995). High expression level of α6 integrin in human breast carcinoma is correlated with reduced survival. Cancer Res. 55:901-906.
E. Tagliabue, C. Ghirelli, P. Squicciarini, P. Aiello, M. I. Colnaghi, and S. Menard (1998). Prognostic value of α6β4 integrin expression in breast carcinomas is affected by laminin production from tumor cells. Clin. Cancer Res. 4:407-410.
U. M. Wewer, L. M. Shaw, R. Albrechtsen, and A. M. Mercurio (1997). The integrin α6β1 promotes the survival of metastatic human breast carcinoma cells in mice. Am. J. Pathol. 151:1191-1198.
R. Mukhopadhyay, R. L. Theriault, and J. E. Price (1999). Increased levels of α6 integrins are associated with the metastatic phenotype of human breast cancer cells. Clin. Exp. Metastasis 17:325-332.
W. G. Stetler-Stevenson, S. Aznavoorian, and L. A. Liotta (1993). Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu. Rev. Cell. Biol. 9:541-573.
E. D. Hay (1995). Anoverview of epithelio-mesenchymal transformation. Acta. Anat. (Basel) 154:8-20.
J. P. Thiery and D. Chopin (1999). Epithelial cell plasticity in development and tumor progression. Cancer Metastasis Rev. 18:31-42.
C. Birchmeier, W. Birchmeier, and B. Brand-Saberi (1996). Epithelial-mesenchymal transitions in cancer progression. Acta Anat. (Basel) 156:217-226.
B. P. Wijnhoven, W. N. Dinjens, and M. Pignatelli (2000). E-cadherin-catenin cell-cell adhesion complex and human cancer. Br. J. Surg. 87:992-1005.
J. Behrens (1999). Cadherins and catenins: Role in signal transduction and tumor progression. Cancer Metastasis Rev. 18:15-30.
W. Birchmeier, J. Hulsken, and J. Behrens (1995). E-cadherin as an invasion suppressor. Ciba Found Symp. 189:124-136.
W. Birchmeier (1995). E-cadherin as a tumor (invasion) suppressor gene. Bioessays 17:97-99.
M. G. Nievers, R. Q. Schaapveld, and A. Sonnenberg (1999). Biology and function of hemidesmosomes. Matrix Biol. 18:5-17.
L. M. Bergstraesser, G. Srinivasan, J. C. Jones, S. Stahl, and S. A. Weitzman (1995). Expression of hemidesmosomes and component proteins is lost by invasive breast cancer cells. Am. J. Pathol. 147:1823-1839.
I. Rabinovitz and A. M. Mercurio (1996). The integrin α6β4 and the biology of carcinoma. Biochem. Cell. Biol. 74:811-821.
C. Chao, M. M. Lotz, A. C. Clarke, and A. M. Mercurio (1996). A function for the integrin α6β4 in the invasive properties of colorectal carcinoma cells. Cancer Res. 56:4811-4819.
H. Sun, S. A. Santoro, and M. M. Zutter (1998). Downstream events in mammary gland morphogenesis mediated by reexpression of the α2β1 integrin: The role of the α6 and β4 integrin subunits. Cancer Res. 58:2224-2233.
K. L. O'Connor, L. M. Shaw, and A. M. Mercurio (1998). Release of cAMP gating by the α6β4 integrin stimulates lamellae formation and the chemotactic migration of invasive carcinoma cells. J. Cell. Biol. 143:1749-1760.
L. Bonaccorsi, V. Carloni, M. Muratori, A. Salvadori, A. Giannini, M. Carini, M. Serio, G. Forti, and E. Baldi (2000). Androgen receptor expression in prostate carcinoma cells suppresses α6β4 integrin-mediated invasive phenotype. Endocrinology 141:3172-3182.
D. A. Lauffenburger and A. F. Horwitz (1996). Cell migration: A physically integrated molecular process. Cell 84:359-369.
I. Rabinovitz and A. M. Mercurio (1997). The integrin α6β4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J. Cell. Biol. 139:1873-1884.
F. Mainiero, A. Pepe, M. Yeon, Y. Ren, and F. G. Giancotti (1996). The intracellular functions of alpha6beta4 integrin are regulated by EGF. J. Cell. Biol. 134:241-253.
I. Rabinovitz, A. Toker, and A. M. Mercurio (1999). Protein kinase C-dependent mobilization of the α6β4 integrin from hemidesmosomes and its association with actin-rich cell protrusions drive the chemotactic migration of carcinoma cells. J. Cell. Biol. 146:1147-1160.
A. E. Aplin, A. K. Howe, and R. Juliano (1999). Cell adhesion molecules, signal transduction and cell growth. Curr. Opin. Cell. Biol. 11:737-744.
P. J. Keely, J. K. Westwick, I. P. Whitehead, C. J. Der, and L. V. Parise (1997). Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature 390:632-636.
A. Toker and L. C. Cantley (1997). Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature 387:673-676.
S. Menard, E. Tagliabue, M. Campiglio, and S. M. Pupa (2000). Role of HER2 gene overexpression in breast carcinoma. J. Cell. Physiol. 182:150-162.
R. Falcioni, A. Antonini, P. Nistico, S. Di Stefano, M. Crescenzi, P. G. Natali, and A. Sacchi (1997). α6β4 and α6β1 integrins associate with ErbB-2 in human carcinoma cell lines. Exp. Cell. Res. 236:76-85.
D. Gambaletta, A. Marchetti, L. Benedetti, A. M. Mercurio, A. Sacchi, and R. Falcioni (2000). Cooperative signaling between α6β4 integrin and ErbB-2 receptor is required to promote phosphatidylinositol 3-kinase-dependent invasion. J. Biol. Chem. 275:10604-10610.
G. G. Borisy and T. M. Svitkina (2000). Actin machinery: Pushing the envelope. Curr. Opin. Cell. Biol. 12:104-112.
S. M. Schoenwaelder and K. Burridge (1999). Bidirectional signaling between the cytoskeleton and integrins. Curr. Opin. Cell. Biol. 11:274-286.
L Mahadevan and P. Matsudaira (2000). Motility powered by supramolecular springs and ratchets. Science 288:95-100.
E. E. Sander, S. van Delft, J. P. ten Klooster, T. Reid, R. A. van der Kammen, F. Michiels, and J. G. Collard (1998). Matrixdependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J. Cell. Biol. 143:1385-1398.
D. H. van Weering, J. de Rooij, B. Marte, J. Downward, J. L. Bos, and B. M. Burgering (1998). Protein kinase B activation and lamellipodium formation are independent phosphoinositide 3-kinase-mediated events differentially regulated by endogenous Ras. Mol. Cell. Biol. 18:1802-1811.
R. Meili, C. Ellsworth, S. Lee, T. B. Reddy, H. Ma, and R. A. Firtel (1999). Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 18:2092-2105.
K. L. O'Connor, B. K. Nguyen, and A. M. Mercurio (2000). RhoA function in lamellae formation and migration is regulated by the α6β4 integrin and cAMP metabolism. J. Cell. Biol. 148:253-258.
C. Laudanna, J. J. Campbell, and E. C. Butcher (1997). Elevation of intracellular cAMP inhibits RhoA activation and integrin-dependent leukocyte adhesion induced by chemoattractants. J. Biol. Chem. 272:24141-24144.
K. Itoh, K. Yoshioka, H. Akedo, M. Uehata, T. Ishizaki, and S. Narumiya (1999). An essential part for Rho-associated kinase in the transcellular invasion of tumor cells. Nat. Med. 5:221-225.
K. Yoshioka, F. Matsumura, H. Akedo, and K. Itoh (1998). Small GTP-binding protein Rho stimulates the actomyosin system, leading to invasion of tumor cells. J. Biol. Chem. 273:5146-5154.
S. M. Frisch and H. Francis (1994). Disruption of epithelial cellmatrix interactions induces apoptosis. J. Cell. Biol. 124:619-626.
N. Farrelly, Y. J. Lee, J. Oliver, C. Dive, and C. H. Streuli (1999). Extracellular matrix regulates apoptosis in mammary epithelium through a control on insulin signaling. J. Cell. Biol. 144:1337-1348.
M. A. Schwartz (1997). Integrins, oncogenes, and anchorage independence. J. Cell. Biol. 139:575-578.
C. Hagios, A. Lochter, and M. J. Bissell (1998). Tissue architecture: The ultimate regulator of epithelial function? Philos. Trans. R. Soc. Lond. B. Biol. Sci. 353:857-870.
J. M. Brown (1999). The hypoxic cell: A target for selective cancer therapy—eighteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 59:5863-5870.
D. Hanahan and J. Folkman (1996). Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86:353-364.
B. R. Zetter (1998). Angiogenesis and tumor metastasis. Annu. Rev. Med. 49:407-424.
S. R. Datta, A. Brunet, and M. E. Greenberg (1999). Cellular survival: A play in three Akts. Genes Dev. 13:2905-2927.
J. M. Shields, K. Pruitt, A. McFall, A. Shaub, and C. J. Der (2000). Understanding Ras: 'it ain't over 'til it's over'. Trends Cell. Biol. 10:147-154.
L. C. Cantley and B. G. Neel (1999). New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. U.S.A. 96:4240-4245.
A. Toker and A. C. Newton (2000). Akt/Protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J. Biol. Chem. 275:8271-8274.
A. Khwaja, P. Rodriguez-Viciana, S. Wennstrom, P. H. Warne, and J. Downward (1997). Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 16:2783-2793.
V. Stambolic, A. Suzuki, J. L. de la Pompa, G. M. Brothers, C. Mirtsos, T. Sasaki, J. Ruland, J. M. Penninger, D. P. Siderovski, and T. W. Mak (1998). Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95:29-39.
J. Downward (1998). Ras signalling and apoptosis. Curr. Opin. Genet. Dev. 8:49-54.
A. Bellacosa, D. de Feo, A. K. Godwin, D. W. Bell, J. Q. Cheng, D. A. Altomare, M. Wan, L. Dubeau, G. Scambia, V. Masciullo, et al. (1995). Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int. J. Cancer 64:280-285.
Z. Q. Yuan, M. Sun, R. I. Feldman, G. Wang, X. Ma, C. Jiang, D. Coppola, S. V. Nicosia, and J. Q. Cheng (2000). Frequent activation of AKT2 and induction of apoptosis by inhibition of phosphoinositide-3-OH kinase/Akt pathway in human ovarian cancer. Oncogene 19:2324-2330.
K. Jiang, D. Coppola, N. C. Crespo, S. V. Nicosia, A. D. Hamilton, S. M. Sebti, and J. Q. Cheng (2000). The phosphoinositide 3-OH kinase/AKT2 pathway as a critical target for farnesyltransferase inhibitor-induced apoptosis. Mol. Cell. Biol. 20:139-148.
K. Nakatani, D. A. Thompson, A. Barthel, H. Sakaue, W. Liu, R. J. Weigel, and R. A. Roth (1999). Up-regulation of Akt3 in estrogen receptor-deficient breast cancers and androgenindependent prostate cancer lines. J. Biol. Chem. 274:21528-21532.
L. M. Shaw, C. Chao, U. M. Wewer, and A. M. Mercurio (1996). Function of the integrin α6β1 in metastatic breast carcinoma cells assessed by expression of a dominant-negative receptor. Cancer Res. 56:959-963.
A. Toker (2000). Protein kinases as mediators of phosphoinositide 3-kinase signaling. Mol. Pharmacol. 57:652-658.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mercurio, A.M., Bachelder, R.E., Chung, J. et al. Integrin Laminin Receptors and Breast Carcinoma Progression. J Mammary Gland Biol Neoplasia 6, 299–309 (2001). https://doi.org/10.1023/A:1011323608064
Issue Date:
DOI: https://doi.org/10.1023/A:1011323608064