Abstract
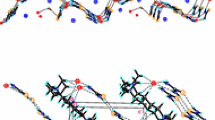
New inclusion complexes (C2H5)4E+Cl-⋅2(NH2)2CO(1, E = N; 2, E = P) have beenprepared and characterized by X-ray crystallography. Crystal data, MoKα radiation: 1, space group P21/c,Z = 4, a = 10.492(6), b = 14.954(8), c = 10.335(6) Å, β = 91.02(5)°, R F = 0.050 for 1527 observed data; 2, space group Pmn21, Z = 2, a = 7.446(1), b = 9.200(2), c = 12.753(3) Å, R F = 0.079 for 519 observed data. In compound 1 the tetraethylammonium ions are sandwiched between puckered layers, which are constructed from the linkage of chloride ions and wide urea ribbons each composed of a broadside arrangement of centrosymmetric hydrogen-bonded urea dimers. In the crystal structure of 2, hydrogen-bonded urea ribbons running parallel to [100] are connected by chloride ions to generate a sawtooth wave layer, and stacked columns of tetraethylphosphonium cations are sandwiched between adjacent layers.
Supplementary Data relating to this article have been deposited with the British Library as Supplementary Publication No. SUP 82216 (11 pages).
Similar content being viewed by others
References
L. C. Fetterly: in L. Mandelcorn (ed.), Non-stoichiometric Compounds, pp 491–567. Academic Press, New York (1964).
K. Takemota and N. Sonoda: in J. L. Atwood, J. E. D. Davies and D. D. MacNicol (eds.), Inclusion Compounds, Vol. 2, pp 47–67. Academic Press, London (1984).
T. W. Bell and J. Liu: J. Am. Chem. Soc. 110, 3673 (1988).
W. Schlenk: Liebigs Ann. Chem. 565, 204 (1949).
A. E. Smith: J. Chem. Phys. 18, 150 (1950).
A. E. Smith: Acta Crystallogr. 5, 224 (1952).
F. Laves, N. Nicolaides, and K. C. Peng: Z. Kristallogr. 121, 258 (1965).
Y. Chatani, Y. Taki and H. Tadokoro: Acta Crystallogr. Sect. B 33, 309 (1977).
Y. Chatani, H. Anraku, and Y. Taki: Mol. Cryst. Liq. Cryst. 48, 219 (1978).
R. Forst, H. Jagodzinski, H. Boysen, and F. Frey; Z. Kristallogr. 174, 56 (1986); 174, 58 (1986).
R. Forst, H. Boysen, F. Frey, H. Jagodzinski, and C. Zeyen: J. Phys. Chem. Solids 47, 1089 (1986).
R. Forst, H. Jagodzinski, H. Boysen, and F. Frey: Acta Crystallogr., Sect. B 43, 187 (1987).
T. C. W. Mak and R. K. McMullan: J. Incl. Phenom. 6, 473 (1988).
W. Pryor and P. L. Sanger: Acta Crystallogr., Sect. A 26, 543 (1970).
D. Rosenstein, R. K. McMullan, D. Schwarzenbach, and G. A. Jeffrey: Am. Crystallogr. Assoc. Abstr. Papers (Summer Meeting), p. 152 (1973).
T. C. W. Mak: unpublished data.
Q. Li and T. C. W. Mak: J. Incl. Phenom. 20, 73 (1995).
Q. Li and T. C. W. Mak: Acta Crystallogr., Sect. B(CR 511).
Q. Li and T. C. W. Mak: J. Incl. Phenom. 27, 319 (1997).
Q. Li and T. C. W. Mak: J. Incl. Phenom.accepted.
H. Schmidbaur, G. Blaschke, B. Zimmer-Gasser, and U. Schubert: Chem. Ber. 113, 1612 (1980).
D. L. Thorn, R. L. Harlow, and N. Herron: Inorg. Chem. 34, 2629 (1995).
W. G. Haije, J. A. L. Dobbelaar, and W. J. A. Maaskant: Acta Crystallogr., Sect. C 42, 1485 (1986).
R. A. Sparks: in F.R. Ahmed (ed.), Crystallographic Computing Techniques, p. 452. Munksgaard, Copenhagen (1976).
G. Kopfmann and R. Huber: Acta Crystallogr., Sect. A 24, 348 (1968).
G. M. Sheldrick: in D. Sayre (ed.), Computational Crystallography, Oxford University Press, New York (1982), pp 506–514.
International Tables for X-ray Crystallography, Vol. IV, Kynoch Press, Birmingham (1974) (Distrib.: Kluwer Academic Publishers, Dordrecht), pp. 55, 99, 149.
Q. Li and T. C. W. Mak: Acta Crystallogr., Sect. C.in press (C960826-KH1101).
Q. Li and T. C.W. Mak: J. Incl. Phenom. 23, 233 (1995).
M. R. Pressprich and R. D. Willet: Acta Crystallogr., Sect. C 47, 1188 (1991).
H. Schmidbaur, G. Muller, B. Milewski-Mahrla, and U. Schubert: Chem. Ber. 113, 2575 (1980).
M. L. Glówka and Z. Galdecki: Acta Crystallogr., Sect. B 36, 2312 (1980).
T. Kovacs and L. Parkanyi: Cryst. Struct. Commun. 11, 1565 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
LI, Q., MAK, T.C.W. Hydrogen-Bonded Urea-Anion Host Lattices. Part 4. Comparative Study of Inclusion Compounds of Urea with Tetraethylammonium and Tetraethylphosphonium Chlorides. Journal of Inclusion Phenomena 28, 151–161 (1997). https://doi.org/10.1023/A:1007985329632
Issue Date:
DOI: https://doi.org/10.1023/A:1007985329632