Abstract
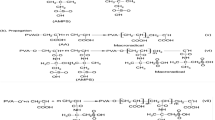
The use of curdlan, a natural β-1,3-glucan, in protein drug delivery vehicles was studied by carrying out in vitro release studies with curdlan gels containing bovine serum albumin (BSA) as a model protein. Addition of urea (8 M) decreased the gel formation temperature to 37 °C. Curdlan was hydroxyethylated in order to form gels under mild conditions such as physiological temperature and pH. In gels formed in 8 M urea solution, urea was almost released after 2 h while BSA was completely released after 45–100 h. The total time for complete release of BSA increased with curdlan concentration within gels. The strength of hydroxyethylated curdlan gels (385.7 dyne cm−2) was weaker than that of curdlan gels formed in 8 M urea solution (6277 dyne cm−2).
Similar content being viewed by others
References
Gombots W, Pettit DK (1995) Biodegradable polymers for protein and peptide drug delivery. Bioconjugate Chem. 6: 332–351.
Harada T (1977) Production, properties, and application of curdlan. In: Sanford PA, Laskin A, eds. Extracellular Microbial Polysaccharides. Washington DC: American Chemical Society, pp. 265–283.
Harada T, Misaka A, Saito H (1968) Curdlan: a bacterial gelforming _-1,3-glucan. Arch. Biochem. Biophys. 124: 292–298.
Jezequel V (1998) Curdlan: a new functional _-glucan. Cereal Foods World 43: 361–364.
Kanke M, Koda K, Koda Y, Katayama H (1992) Application of curdlan to controlled drug delivery. I. The preparation and evaluation of theophylline-containing curdlan tablets. Pharm. Res. 9: 414–418.
Kanke M, Katayama H, Nakamura M (1995a) Application of curdlan to controlled drug delivery. II. In vitro and in vivo drug release studies of theophylline-containing curdlan tablets. Biol. Pharm. Bull. 18: 1104–1108.
Kanke M, Tanabe E, Katayama H, Koda Y, Yoshitomi H (1995b) Application of curdlan to controlled drug delivery. III. Drug release from sustained release suppositories in vitro. Biol. Pharm. Bull. 18: 1154–1158.
Langer R (1998) Drug delivery and targeting.Nature 392 (6679 Suppl): 5–10.
Renn DW (1997) Purified curdlan and its hydroxyalkyl derivatives: preparation, properties and applications. Carbohydr. Polym. 33: 219–225.
Watanabe K, Yakou S, Takayama K, Machida T, Nagai T (1992) Factors affecting prednisolone release from hydrogels prepared with water-soluble dietary fibers, xanthan and locust bean gums. Chem. Pharm. Bull. 40: 459–462.
West JL, Hubbell JA (1995) Photopolymerized hydrogel materials for drug delivery applications. Reactive Polym. 25: 139–147.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, B.S., Jung, I.D., Kim, J.S. et al. Curdlan gels as protein drug delivery vehicles. Biotechnology Letters 22, 1127–1130 (2000). https://doi.org/10.1023/A:1005636205036
Issue Date:
DOI: https://doi.org/10.1023/A:1005636205036