Abstract
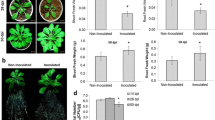
Hyphal branches of the primary germ tubes and secondary hyphae of Gigaspora gigantea, Gigaspora rosea, and Glomus intraradices were induced by exposure to light. The photo- induced branching of G. rosea was increased if the germinated spores were first grown in the presence of 10 μM quercetin before exposure to light. Further analyses with G. gigantea showed that at low intensity light (13.4 μE s-1m-2), maximum branching was achieved after a 6 h exposure and at high intensity light (10,800 μE s-1m-2), maximum branching was reached after an 8 min exposure. Multiple exposures to alternating low light followed by a dark incubation period indicated that the photo-effect was not additive. Photo-induced branching did not need a subsequent dark period for the growth of hyphal branches because branching occurred during prolonged continuous light. The light-induced branching appeared to have ecological relevance. Corn seedlings (Zea maize L.) grown in AM fungal inocula exposed to light had a higher percentage of their root system colonized by G. gigantea than those in inocula that remained in the dark.
Similar content being viewed by others
References
Bécard G, Beguiristain T and Nagahashi G 1997 Signaling in plants and root-infecting fungi associations. In Radical Biology: Advances and Perspectives on the Function of Plant Roots. Eds HE Flores, JP Lynch and D Eissenstat. Vol 18 pp 164–177. Current Topics in Plant Physiology. American Society of Plant Physiologists, publisher.
Bécard G, Douds D D, Jr and Pfeffer P E 1992 Extensive in vitro hyphal growth of vesicular-arbuscular mycorrhizal fungi in the presence of CO2 and flavonols. Appl. Envir. Microbiol. 58, 821–825.
Bécard G and Fortin J A 1988 Early events of vesicular-arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytologist 108, 211–218.
Corrochano L M and Cerda-Olmedo E 1991 Photomorphogenesis in Phycomyces and in other fungi. Photochemistry and Photobiology 54, 319–327.
Douds D D, Galvez L, Franke-Snyder M, Reider C and Drinkwater L E 1997 Effect of compost addition and crop rotation point upon Vam fungi. Agriculture, Ecosystems and Environment 65, 257–266.
Douds D D, Nagahashi G and Abney G 1996. The differential effects of cell wall-associated phenolics, cell walls and cytosolic phenolics of host and non-host roots on the growth of two species of AM fungi. New Phytologist 133, 289–294.
Galland P 1992 Forty years of blue-light research and no anniversary. Photochemistry and Photobiology 56, 847–853.
Gressel J and Rau W 1983 Photocontrol of Fungal Development. In Encyclopedia of Plant Physiology. Eds W. Shropshire Jr and H. Mohr. Vol 16B pp 603–639. Photomorphogenesis, Springer-Verlag, Heidelberg, New York.
Horwitz B A and Berrocal G M 1997 A spectroscopic view of some recent advances in the study of blue light photoreception. Botanica Acta 110, 360–368.
Horwitz B A and Gressel J 1987 First measurable effects following photoinduction of morphogenesis. In Blue Light Responses. Ed. Vol II. pp 53–70. Phenomena and Occurrence, H. Senger ed. CRC Press, Inc.
Lauter F-R, Marchfelder U, Russo V E A, Yamashiro C T, Yatzkan E and Yardens O 1998 Photoregulation of cot-1, a kinase-encoding gene involved in hyphal growth in Neurospora crassa. Fungal Genet. Biol. 23, 300–310.
Nagahashi G, Douds D D and Bécard G 1999 Recognition and communication events between arbuscular mycorrhizal fungi and host roots. Current Topics in Plant Biology 1, 63–75.
Newman E I 1966 A method of estimating the total length of root in a sample. J. Appl. Ecol. 3, 139–145.
Phillips J M and Hayman D S 1970 Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society 55, 158–160.
St. -Arnaud M, Hamel C, Vimard B, Caron M and Fortin J A 1996 Enhanced hyphal growth and spore production of the arbuscular mycorrhizal fungus Glomus intraradices in an in vitro system in the absence of host roots. Mycological Res. 100, 328–332.
Tester M and Morris C 1987 The penetration of light through soil. Plant Cell Environ. 10, 281–286.
Varela-Castejon C, Gonzalez-Penalta B, Vilarino A and Sainz M J 1998 Fluorescent light inhibits the germination of propagules of the arbuscular mycorrhizal fungus Glomus macrocarpum. Soil Biol. Biochem. 30, 1845–1847.
Rights and permissions
About this article
Cite this article
Nagahashi, G., Douds, D. & Buee, M. Light-induced hyphal branching of germinated AM fungal spores. Plant and Soil 219, 71–79 (2000). https://doi.org/10.1023/A:1004714530021
Issue Date:
DOI: https://doi.org/10.1023/A:1004714530021