Abstract
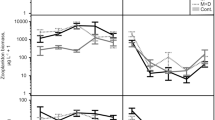
The superiority of large zooplankton in suppressingphytoplankton growth has often been inferred from theSize Efficiency Hypothesis (S.E.H.). The S.E.H. hasoriginally been formulated to account for thecompetitive superiority of large to small zooplanktonunder food limiting conditions. Extrapolation of itspredictions to the suppression of phytoplankton byzooplankton under high food availability, should bedone with care. In an attempt to assess the relevanceof the S.E.H. to biomanipulation theory inhypertrophic systems, a fish exclosure experiment wascarried out in which the efficiency of two differentlystructured zooplankton communities in reducingphytoplankton biomass was examined. By inoculatingpart of the enclosures with laboratory grownDaphnia magna, a community dominated by this largecladoceran species could be compared with a communitymainly consisting of Bosmina and smallerDaphnia species. After the exclusion of fish, therewas an exponential increase of total zooplanktonbiomass. Phytoplankton growth was efficientlysuppressed to equal levels in both treatments, thoughthere was a difference in timing: chlorophyll-a levelsin the enclosures inoculated with D. magnadropped one week earlier than in non-inoculatedenclosures. The time-lag was even more pronounced whenlarge phytoplankton was considered. In accordance withthe S.E.H., the time lags could be explained bydifferences in population growth potential as well asby differences in zooplankton grazing rates(indirectly measured as the minimal zooplanktonbiomass needed to suppress phytoplankton growth) andfood particle size range.
Similar content being viewed by others
References
Bergquist, A. M. & S. R. Carpenter, 1986. Limnetic herbivory: effects on phytoplankton populations and primary production. Ecology 67: 1351–1360.
Bergquist, A. M., S. R. Carpenter & J. C. Latino, 1985. Shifts in phytoplankton size structure and community composition during grazing by contrasting zooplankton assemblages. Limnol. Oceanogr. 30: 1037–1045.
Bottrell, H. H., A. Duncan, Z. M. Gliwicz, E. Grygierek, A. Herzig, A. Hillbricht–Ilkowska, H. Kurasawa, P. Larsson & T. Weglenska, 1976. A review of some problems in zooplankton production studies. Norw. J. Zool. 24: 419–456.
Brett, M. T. & C. R. Goldman, 1997. Consumer versus resource control in freshwater pelagic food webs. Science 275: 384–386.
Brooks, J. H. & S. I. Dodson, 1965. Predation, body size, and composition of plankton. Science 150: 28–35.
Carpenter, S. R. & J. F. Kitchell, 1992. Trophic cascade and biomanipulation: Interface of research and management-A reply to the comment by DeMelo et al. Limnol. Oceanogr. 371: 208–213.
Carpenter, S. R., J. F. Kitchell & J. R. Hodgson, 1985. Cascading trophic interactions and lake productivity. BioScience 35: 634–638.
Christoffersen, K., B. Riemann, A. Klysner & M. Sondergaard, 1993. Potential role of fish predation and natural poopulations of zooplankton in structuring a plankton community in eutrophic lake water. Limnol. Ocanogr. 38: 561–573.
Dawidowicz, P., 1990. Effectiveness of phytoplankton control by large-bodied and small-bodied zooplankton. Hydrobiologia 200/201: 43–47.
De Meester, L., 1996a. Evolutionary potential and local genetic differentiation in a phenotypically plastic trait of a cyclical parthenogen, Daphnia magna. Evolution 50: 1293–1298.
De Meester, L., 1996b. Local genetic differentiation and adaptation in freshwater zooplankton populations: Patterns and processes. Ecoscience 3: 385–399.
DeMelo, R., R. France & D. J. McQueen, 1992. Biomanipulation: Hit or myth? Limnol. Oceanogr. 37: 192–207.
DeMott, W. R. & W. C. Kerfoot, 1982. Competition among cladocerans: nature of the interaction between Bosminaand Daphnia. Ecology 63: 1949–1966.
De Smedt, P., W. Rommens, S. Declerck, W. Vyverman, C. Belpaire, B. Denayer, L. De Meester, F. Ollevier & J. Van Assche, 1997. Ecologisch onderzoek in en rond het erkend natuurreservaat ‘De Blankaart’, met inbegrip van Actief Biologisch Beheer van Kasteel-enVisvijver. Studieopdracht van AMINAL (afdeling Natuur) & Ecologisch Impulsgebied Ijzervallei.
Fussmann, G., 1996. The importance of crustacean zooplankton in structuring rotifer and phytoplankton communities: an enclosure study. J. Plankt. Res. 18: 1897–1915.
Gliwicz, Z. M., 1990a. Food thresholds and body size in cladocerans. Nature 343: 638–640.
Gliwicz, Z. M., 1990b. Why do cladocerans fail to control algal blooms? Hydrobiologia 200/201: 83–97.
Gliwicz, Z. M. & W. Lampert, 1990. Food thresholds in Daphnia species in the absence and presence of blue-green filaments. Ecology 71: 691–702.
Greenberg, A. E., R. R. Trussell & L. S. Clesceri (eds), 1985. Standard Methods for the Examination of Water and Wastewater. APHA, AWWA, WPCF, 16th edn., Washington, U.S.A.
Gulati, R. D., E. H. R. R. Lammens, M.-L. Meijer & E. Van Donk (eds), 1990. Biomanipulation–Tool for Water Management. Dev. Hydrobiol. 61. Kluwer Academic Publishers, Dordrecht, 628 pp. Reprinted from Hydrobiologia 200/201. 200/201: 619–627.
Hall, D. J., S. T. Threlkeld, C.W. Burns & P. H. Crowley, 1976. The size-efficiency hypothesis and the size structure of zooplankton communities. Ann. Rev. Ecol. Syst. 7: 177–208.
Havens, K. E., 1993. An experimental analysis of macrozooplankton, microzooplankton and phytoplankton interactions in a temperate eutrophic lake. Arch. Hydrobiol. 127: 9–20.
Lammens, E. H. R. R., R. D. Gulati, M.-L. Meijer & E. Van Donk, 1990. The first biomanipulation conference: a synthesis. Hydrobiologia 200/201: 619–627.
Massart, J., 1907. Essai de géographie botanique des districts littoraux et alluviaux de la Belgique. Henri Lamertin, Brussel.
O.E.C.D. Eutrophisation des eaux. Méthodes de surveillance, d’évaluation et de lutte. O.E.C.D., Paris.
Peeters, B., L. De Meester, B. Denayer, P. De Smedt, K. Nuydens & F. Ollevier, 1996. Inventarisatie van het visbestand van de Blankaartvijver en omliggende waterlopen met afvissing van de Kasteel-en Visvijver. Beschrijving van de visstand en voorstellen inzake actief biologisch beheer. Studieopdracht van AMINAL (afdeling Natuur) & Ecologisch Impulsgebied Ijzervallei.
Reynolds, C. S., 1994. The ecological basis for successful biomanipulation of aquatic communities. Arch. Hydrobiol. 130: 1–33.
Sarnelle, O., 1993. Herbivore effects on phytoplankton succession in a eutrophic lake. Ecol. Monogr. 63: 129–149.
Scheffer, M., S. H. Hosper, M.-L. Meijer, B. Moss & E. Jeppesen, 1993. Alternative equilibria in shallow lakes. Trends Ecol. Evol. 8: 275–279.
Talling, J. F. & D. Driver, 1963. Some problems in the estimation of chlorophyll-ain phytoplankton. Proc. Conference on Primary Productivity Measurements, Marine and Freshwater. US Atomic Energy. Comm. TID7633: 142–146.
Tessier A. J. & C. E. Goulden, 1987. Cladoceran juvenile growth. Limnol. Oceanogr. 32: 680–686.
Threlkeld, S. T., 1988. Planktivory and planktivore biomass effects on zooplankton, phytoplankton, and the trophic cascade. Limnol. Oceanogr. 33: 1362–1375.
Uehlinger, U., P. Bossard, J. Bloesch, H. R. Bürgi & H. Bührer, 1984. Ecological experiments in limnocorrals: Methodological problems and quantification of the epilimnetic phosphorus and carbon cycles. Verh. int. Ver. Limnol. 22: 163–171.
UNESCO, 1974. A review of methods used for qualitative phytoplankton studies. Unesco tec. Pap. Mar. Sci. 18.
Van der Werf, B., J. Schrotenboer, A. F. Richter, J. R. Moed, H. L. Hoogveld & H. De Haan, 1987. A durable and transportable limnetic enclosure system suitable for windexposed lakes. Can. J. Fish. aquat. Sci. 44: 1649–1652.
Van Donk, E., M. P. Grimm, P. G. M. Heuts, G. Blom, K. Everards & O. F. R. van Tongeren, 1994. Use of mesocosms in a shallow eutrophic lake to study the effects of different restoration measures. Arch. Hydrobiol. Beih. Ergebn. Limnol. 40: 283–294.
Vanni M. J. & C. D. Layne, 1997. Nutrient recycling and herbivory as mechanisms in the ‘top-down’ effect of fish on algae in lakes. Ecology 78: 21–40.
Vanni M. J. & D. L. Findlay, 1990. Trophic cascades and phytoplankton community structure. Ecology 71: 921–937.
Vanni, M. J., C. D. Layne & S. E. Arnott, 1997. ‘Top-down’ trophic interactions in lakes: effects of fish on nutrient dynamics. Ecology 78: 1–20.
Zaret, T. M., 1980. Predation and Freshwater Communities. Yale University Press.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Declerck, S., De Meester, L., Podoor, N. et al. The relevance of size efficiency to biomanipulation theory: a field test under hypertrophic conditions. Hydrobiologia 360, 265–275 (1997). https://doi.org/10.1023/A:1003175929208
Issue Date:
DOI: https://doi.org/10.1023/A:1003175929208