Introduction
Post-traumatic stress disorder (PTSD) typically arises following exposure, both direct and indirect, to a traumatic event, and is characterized by the onset and persistence of a series of clinical symptoms that can often be profoundly incapacitating and tendentially chronic. 1 There is evidence that PTSD is a chronic, drug-resistant disorder, associated with high suicidal risk (about 25% of patients have attempted suicide), substance abuse, and maladaptive behaviors, that can also be insidious beginning even after years of silence.Reference Sareen, Cox and Stein 2 The lifetime prevalence of PTSD can vary considerably depending on the populations considered and the year in which the study was carried out for the adopted diagnostic criteria. Nowadays, the prevalence in the general population corresponds to about 8%, that can arise up to even 40% in aftermath of a natural disaster, depending on its severity and impact on exposed populations, with twice or much of the rates in women compared to men.Reference Kessler, Sonnega and Bromet 3 , Reference Carmassi, Dell’Osso and Manni 4
In recent years, several studies have examined and confirmed the strict relationship between mental disorders and physical health. Suffering from mental disorder, in fact, may determine biochemical and hormonal alterations in the body that can lead to various physical illnesses. In particular, stressful life events and PTSD have been closely correlated with various physical disorders, especially somatic symptoms such as chronic pain, gastrointestinal (GI) disorders, and headache.Reference Barkmann, Braehler and Schulte-Markwort5, Reference Pacella, Hruska and Delahanty 6 High rates of somatic symptoms have been reported among war-exposed civilians,Reference Morina, Ford and Risch 7 , Reference Cheung, Makhashvili and Javakhishvili 8 as well as in special populations, who regularly exposed to traumatic events because of their employment states, such as military veterans and emergency services personnel.Reference Pacella, Hruska and Delahanty 6 , Reference Hoge, Terhakopian and Castro 9 , Reference Milligan-Saville, Paterson and Harkness 10 Increasing data highlighted the importance to detect full and partial PTSD in general populations exposed to natural disasters in order to provide effective support and treatment, but despite the emerging evidence on a growing use of painkillers in such populations even months after exposure,Reference Angeletti, Guetti and Papola 11 –Reference Carmassi, Rossi and Pedrinelli 13 scant data explored the onset of somatic severe symptoms. The studies available investigated special populations, such as children and adolescents, exposed to natural disasters such as earthquake. In particular, Zhang et alReference Zhang, Zhu and Du 14 investigated somatic symptoms in 2299 children and adolescent survivors of the Lushan 2013 earthquake with probable PTSD, demonstrating a strong association between PTSD and somatic symptoms.
The exact mechanism underlying the link between PTSD and somatic symptoms have not yet been identified though they are believed to be the result of both biological and psychological changes occurring after the exposure to a traumatic event.Reference Schnurr and Green 15 , Reference Miao, Chen and Wei 16 PTSD is characterized by a failure to physiologically adapt to stressors and reminders of the stressors, such that the long-term activation of the stress pathways like the sympathetic nervous system and hypothalamic-pituitary-adrenal (HPA) axis with consequent influence on immune activity that places individuals at higher risk for developing or exacerbating physical medical conditions.Reference Schnurr and Jankowski 17 –Reference Dougall, Baum, Schnurr and Green 19
A link between chronic pain and PTSD or trauma exposure has in fact been hypothesized to be underlined by a chronic altered cortisol or inflammatory response.Reference Boscarino 18 , Reference Kendall-Tackett 20 In a variety of samples, most of the literature regarding the relationship between PTSD and chronic pain indicates that individuals with PTSD report greater frequency or severity of overall pain than those without PTSD.Reference Kendall-Tackett 21 –Reference Smith, Shipherd and Schuster 23 More specifically, these conditions include mainly fibromyalgia, back pain and headaches, and arthritis pain.Reference Sareen, Cox and Stein 2 , Reference Vedantham, Brunet and Boyer 24 –Reference Kelly 28
A positive relationship between PTSD and GI problems, including irritable bowel syndrome (IBS), vomiting, ulcer, and constipation/diarrhea has also been demonstrated.Reference Sareen, Cox and Stein 2 , Reference Lauterbach, Vora and Rakow 27 –Reference Schnurr, Spiro and Paris 31 Further studies analyzed the relationship between migraine and PTSD, suggesting PTSD to be more common in patients with migraine than in the general population.Reference Saunders, Merikangas and Low 32
There is evidence that PTSD usually occurs almost twice as much in women compared to men and this has been also confirmed among general population exposed to earthquake.Reference Dell’Osso, Carmassi and Massimetti 33 –Reference Dell’Osso, Carmassi and Rucci 34 However, little or no data are currently available on the possible gender differences in somatic symptoms emerging in patients with PTSD in the aftermath of trauma exposure. A first study from McCall-Hosenfeld et alReference McCall-Hosenfeld, Winter and Heeren 35 in a sample of 597 urban primary care patients with chronic pain showed women to report significantly more somatic symptoms than men when exposed to three interpersonal trauma types (sexual trauma, intimate partner violence, and childhood trauma history), although somatic symptoms were increased among all interpersonal trauma survivors. More recently, Morina et alReference Morina, Schnyder and Klaghofer 36 showed women reported significantly higher levels of somatic symptoms than men in sample of 142 civilian war survivors, whereas levels of PTSD symptoms were similar in both the genders. Conversely, a systematic review including 26 studies showed that individuals who reported exposure to trauma, especially men, reported an increased prevalence of functional somatic syndromes. The magnitude of the association of these latters with PTSD was significantly stronger than that with trauma exposure alone; however, the gender differences lost the statistical significance by performing more conservative analyses in the same sample.Reference Afari, Ahumada and Wright 37
In light of these data, the aim of the present study was to investigate the possible impact of gender and PTSD on the onset of somatic symptoms, complained in the aftermath of the event, among young adult survivors of the 2009 L’Aquila earthquake in Italy.
Methods
Participants
The study sample consisted in 450 high-school senior students, 253 (56.2%) male and 197 (47.8%) female (mean age ± SD: 17.64 ± 0.78 years), who had been exposed to the 2009 L’Aquila earthquake, enrolled 21 months after the event. Sociodemographic characteristics of the study sample were detailed elsewhere.Reference Carmassi, Akiskal and Yong 38 PTSD was diagnosed, according to DSM-5 criteria, in 162 (36.0%) subjects, specifically in 61 men and 101 women (24.1% vs 51.3%, P < .001).
The Ethics Committee of the University of L’Aquila and the school councils approved all recruitment and assessment procedures. In accordance with the Declaration of Helsinki, subjects provided written informed consent after receiving a complete description of the study.
Instruments and assessments
Subjects were assessed by the modified versions of the Mood Spectrum Self-Report-Lifetime Version (MOODS-SR) and the Trauma and Loss Spectrum Self-Report (TALS-SR),Reference Dell’Osso, Armani and Rucci 39 , Reference Dell’Osso, Carmassi and Rucci 40 to evaluate symptoms occurred in the aftermath of the earthquake exposure.
The MOODS-SR is a 140-item questionnaire exploring mood spectrum symptoms, coded dichotomously, as present or absent, for one or more periods of at least three to five days.Reference Dell’Osso, Armani and Rucci 39 According to the aims of the present study, we adopted a modified version of the instrument assessing symptoms developed in the aftermath of earthquake. Items are organized into three manic and three depressive domains, exploring “mood,” “energy,” and “cognition,” besides a rhythmicity and vegetative functions domain. This latter explores alterations in the circadian rhythms and vegetative functions, including changes in energy, physical wellbeing, mental and physical efficiency related to the weather and season, and changes in appetite, sleep, and sexual activities across 29 items. Five of the MOODS-SR items belonging to the rhythmicity and vegetative functions domain, assessing changes in somatic symptoms, were examined: item n = 159a (…you repeatedly had distressing physical symptoms, for instance: frequent headaches?), item n = 159b (…you repeatedly had distressing physical symptoms, for instance: your mouth felt dry?), item n = 159c (…you repeatedly had distressing physical symptoms, for instance: you were constipated?), item n = 159d (…you repeatedly had distressing physical symptoms, for instance: you had nausea or other stomach or bowel problems?), item n = 160 (…you were more sensitive or less sensitive than usual to heat, cold or pain?). As we adopted a modified version of the MOODS-SR, as described before, we assessed these symptoms when occurring in the aftermath of earthquake exposure.
The TALS-SR includes 116 items exploring the lifetime experience of a range of loss and/or traumatic events and lifetime symptoms, behaviors and personal characteristics that might represent manifestations and/or risk factors for the development of a stress response syndrome.Reference Dell’Osso, Carmassi and Rucci 40 The instrument is organized into nine domains, of which four exploring PTSD symptomatology, namely, re-experiencing, avoidance and numbing, maladaptive coping, and arousal. A modified TALS-SR version, which specifically evaluated PTSD symptomatology related to the earthquake exposure, was used. According previous studies,Reference Carmassi, Akiskal and Yong 38 , Reference Dell’osso, Stratta, Conversano, Massimetti, Akiskal, Akiskal, Rossi and Carmassi 41 DSM-5 PTSD diagnosis was assessed utilizing the following matching between symptom criteria and TALS-SR items: criterion B (B1 = 80; B2 = 77; B3 = 79; B4 = 78; and B5 = 81); criterion C (C1 = 86; C2 = 87 and/or 88 and/or 89); criterion D (D1 = 90; D2 = 95; D3 = 85; D4 = 96; D5 = 91; D6 = 93; and D7 = 92); and criterion E (E1 = 108; E2 = 99 and/or 100 and/or 102 and/or 103 and/or 104;E3 = 106; E4 = 107; E5 = 105; and E6 = 109). Due to the sample characteristics, criterion A was considered satisfied.
Statistical analysis
Chi-square tests (or Fisher when appropriate) were computed in order to compare endorsement rates of each MOODS-SR somatic symptom item in male vs female and PTSD vs NO-PTSD survivors. To study all the possible effects of gender and PTSD diagnosis (and their possible interaction) on the presence of at least one MOODS-SR somatic symptom item a Decision Tree procedure based on the Chi-squared automated interaction detection growing method was utilized.
Finally, the effects of gender and PTSD on the total MOODS-SR somatic symptoms score (derived by the sum of the scores of item 159a, 159b, 159c, 159d, and 160), and of their possible interactions were analyzed by a model of a two-way analysis of variance (ANOVA).
In order to study the post-traumatic stress symptomatology that is more strongly related to the endorsement of at least one MOODS-SR somatic symptom, and the possible impact of gender, we conducted a multivariate logistic regression that reported the presence of at least one MOODS-SR somatic symptom as a dependent variable and gender and the four TALS-SR domains exploring post-traumatic stress symptoms as independent variables.
All statistical analyses were carried out using the Statistical Package for Social Science (SPSS Inc., Chicago, IL), version 25.0.
Results
Full data on the MOODS-SR were available for 450 survivors, the same that also fulfilled the TALS-SR. MOOD-SR somatic symptoms scores were significantly higher in survivors with PTSD than in those without, except for the MOOD-SR item n = 159b (…you repeatedly had distressing physical symptoms, for instance: your mouth felt dry?). PTSD survivors also showed higher ratio of endorsing at least one MOODS-SR somatic symptom than those without (Table 1). No gender differences emerged in MOODS-SR somatic symptoms, albeit females reported higher rates in each item as well as a statistically significant higher rate of endorsement of at least one MOODS-SR somatic symptom compared to males (Table 2).
Table 1. Comparison of MOODS-SR Somatic Symptom Items Endorsement Rates among L’Aquila Earthquake Young Adult Survivors With (n = 162) and Without (n = 288) PTSD.

Table 2. Gender Differences in MOODS-SR Somatic Symptoms Items Endorsement Rates among L’Aquila Earthquake Young Adult Survivors (Male = 253 and Female = 197).
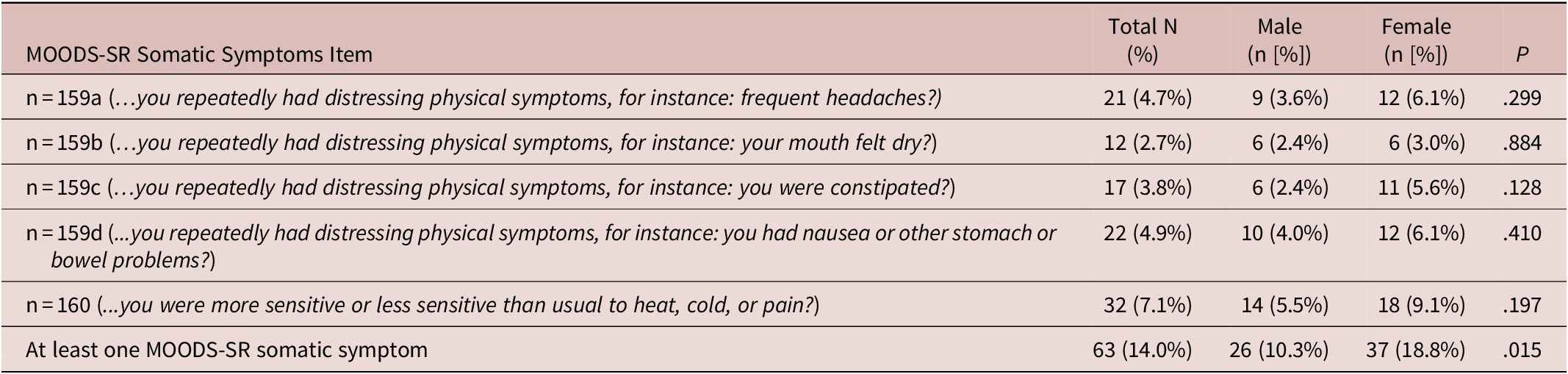
A Decision Tree procedure was utilized to locate the strongest interactions with at least one MOODS-SR somatic symptom (considered as dependent or target variable). The tree-based classification model, able to predict the values of the dependent variable based on the values of the independent ones (gender and PTSD in this case), had an acceptable misclassification risk of the model (value = 0.14). Subjects with PTSD showed a significantly higher ratio of endorsing at least one MOODS-SR somatic symptom compared to those without (25.3% vs 7.6%, χ2 = 26.89, P < .001). According to this model, a significant impact of the gender on the presence of at least one MOODS-SR somatic symptom did not emerged. See Figure 1 for details.

Figure 1. Decision Tree showing post-traumatic stress disorder (PTSD) interaction with Mood Spectrum Self-Report-Lifetime Version (MOODS-SR) somatic symptoms as target variable.
The two-way ANOVA model confirmed only a significant main effect on the total MOODS-SR somatic symptoms score of PTSD [F(1446) = 13.40, P < .001) with a mean score significantly higher in PTSD group compared to No PTSD group (0.40 ± 0.58 vs 0.14 ± 0.54). No significant main effect of gender [F(1446) = 0.48, P = .489], nor of gender × PTSD interaction [F(1446) = 0.43, P = .514], emerged.
The multivariate logistical regression showed a significant association between the presence of at least one MOOD-SR somatic symptom and re-experiencing (odds ratio = 1.37, P = .001) and maladaptive coping (odds ratio = 1.26, P = .029) TALS-SR symptomatologic domains (Table 3).
Table 3. Multivariate Logistic Regression Reporting the Presence of at least One MOODS-SR Somatic Symptom as Dependent Variable and Gender and the Four TALS-SR Domains Exploring Post-Traumatic Stress Symptoms as Independent Variables.
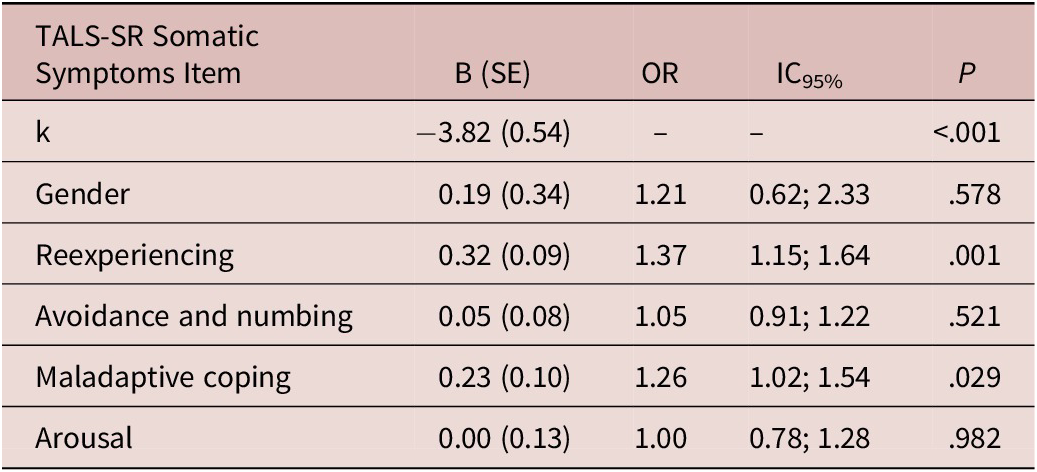
Hosmer and Lemeshow test: χ2 = 8.60, P = .377; Cox and Snell R 2 = 0.10; Nagelkerke R 2 = 0.18 overall percentage of correct classification: 85.3%.
Abbreviations: OR, odds ratio; TALS-SR, Trauma and Loss Spectrum Self-Report; SE, standard error.
Discussion
To the best of our knowledge, the present study is the first to investigate the impact of PTSD and gender on somatic symptoms among civilian young adult survivors to a massive earthquake. According to our data, up to 14% of survivors to the L’Aquila 2009 earthquake complained of somatic symptoms after 21 months from exposure. Complaints included headaches, GI problems, and thermal/painful stimuli hypo- or hypersensitivity, with PTSD survivors showing significantly higher prevalence of such symptoms compared with subjects without PTSD.
In this regard, our results are in line with prior evidence reporting a relationship between PTSD and somatic symptoms, such as headache and stomach pain, in children and adolescents exposed to the Lushan 2013 earthquake.Reference Zhang, Zhang and Zhu 42 In particular, 4.7% of the total sample reported frequent headaches, with statistically significant higher rates, more than two times, in patients with PTSD compared with those without. Growing evidence suggests a relationship between PTSD symptoms and headaches. Smitherman et al,Reference Smitherman and Kolivas 43 in a cross-sectional study on 1051 young adults, compared patients with and without migraine, showing PTSD to be a predictor of migraine. Among veterans, PTSD has been repeatedly associated with increased consumption of medications for headaches, more in men than in women.Reference Seng, Driscoll and Brandt 44 Furthermore, subjects with migraine and comorbid PTSD were found to report a greater disability and more lost workdays due to physical or mental health or substance abuse, and more difficulties with developing and maintaining a social life than subjects with migraine but without PTSD.Reference Peterlin, Tietjen and Brandes 45 , Reference Rao, Scher and Vieira 46 On the other hand, it has also been pointed out that patients with migraine may suffer from lower resilience and subsequent maladaptive stress responses, which might predispose them to PTSD.Reference Peterlin, Nijjar and Tietjen 47 Current hypotheses on neurobiological mechanism underlying the association between PTSD and migraines include serotonergic, autonomic nervous system, and HPA axis dysfunction.Reference Juang and Yang 48
The results of the present study also showed 3.8% of total sample to report constipation and 4.9% nausea or other bowel problems, with almost twice as much rates in PTSD subjects compared with those without. The GI distress related to PTSD has been hypothesized to be linked to the hyperarousal response that is usually associated with enhanced cortical arousal, which can elicit sensations in the gut.Reference Levy and Krebs 49 , Reference Mayer, Bradesi and Chang 50 About 36% of patients seeking treatment for IBS met the criteria for PTSD.Reference Irwin, Falsetti and Lydiard 51 Recently, one study correlated neuropeptide Y (NPY) levels with bowel function in patients with PTSD; in particular, low levels of NPY were reported in the cerebrospinal fluid and plasma of male combat veterans with PTSD, and this is negatively related with sympathetic nervous system (SNS) hyperreactivity, PTSD symptoms, and time to recovery.Reference Rasmusson 52 It should be noted that NPY regulation has not yet been evaluated in women with PTSD. NPY levels are reported to be low in bowel tissue of IBS subjects with diarrhea (IBS-D) vs IBS subjects with constipation. Greater density of ghrelin-containing cells of the gastric oxyntic mucosa has been reported in IBS-D. PTSD-related SNS hyperreactivity may interact with this mechanism to increase ghrelin release, which activates receptors in the lumbosacral spinal cord and basolateral amygdala increasing colonic motility and amygdala hyperreactivity, respectively. Loss of function in the adrenergic a2-autoreceptors gene and increased corticotropin-releasing hormone, as observed in PTSD, are also thought to contribute to IBS-D.Reference Rasmusson 52 , Reference Dell’Osso, Carmassi and Mucci 53
Our data also showed that 7.1% of the total sample reported thermal/painful stimuli hypo- or hypersensitivity, which represents a dysregulation leading to chronic pain. Also in this case, statistically significant higher rates emerged in PTSD subjects than in those without. As previously reported in an Italian study concerning pain in survivors of L’Aquila 2009 earthquake, a high prevalence of somatic conditions such as headaches or lower back pain was detected in the five weeks after the event, associated to increased use of painkillers,Reference Angeletti, Guetti and Papola 11 albeit in such a close observational frame time from the traumatic event, acute symptoms related to wounds or direct damage from the earthquake had affected the results. However, in our findings, the strong relationship between post-traumatic stress and somatic symptoms was well evident after 21 months from the devastating event and related to changing in the perception of somatic health in the aftermath of the event. Further, subjects with PTSD showed more severe chronic pain and this latter correlated with post-traumatic stress symptoms.Reference Defrin, Ginzburg and Solomon 54 , Reference Bartel, Jordan and Correll 55 Pain and PTSD are associated with similar neurobiological abnormalities,Reference Scioli-Salter, Forman and Otis 56 , Reference Dell’Osso, Da Pozzo and Carmassi 57 as hyperarousal,Reference Daniels, McFarlane and Bluhm 58 dysregulations in stress responses,Reference McLean, Clauw and Abelson 59 and in pain modulation.Reference Kosek, Ekholm and Hansson 60 PTSD subjects show an intense and widespread chronic pain and a peculiar sensory profile of hyposensitivity to pain, related to increased dissociation, accompanied by hyperreactivity to suprathreshold noxious stimuli, linked to increased anxiety sensitivity.Reference Defrin, Schreiber and Ginzburg 61 Chronic pain, identified as a persistent condition with an emotional impact, becoming in itself a self-perpetuating stressing event.Reference Chapman and Song 62 , Reference Carmassi, Dell’Oste and Ceresoli 63 Depending on the population studied and the disorder initially assessed, pain can contribute to the onset of PTSD and also maintain it. The same thing is true for PTSD, which can act as both a triggering factor and a maintenance factor of chronic pain.Reference Brennstuhl, Tarquinio and Montel 64
It is also important to recall that in southern Mediterranean populations, somatic symptoms have been reported to be the prevalent manifestation of distress related to trauma.Reference Hiar, Thomas and Hinton 65 The role of somatic complaints after trauma exposure on decreased quality of life seems consistent with the data suggesting that somatization may be a preferred mode of suffering expression in certain populations, thus playing the role of idioms of distress.Reference Kirmayer, Marsella, Friedman, Gerrity and Scurfield 66 –Reference Hinton and Lewis-Fernández 68 This suggests that assessing somatic complaints after trauma exposure, in addition to PTSD symptoms, may help better evaluate the real impact of trauma in exposed populations.
To the best of our knowledge, our results first report on the gender differences in somatic complaints among earthquake survivors in the general population. In particular, no significant impact of gender seemed to emerge on the risk of developing somatic symptoms in the aftermath of a non-war-related massive traumatic event. Despite the fact that we found statistically significant higher rates of at least one MOODS-SR somatic symptom in females rather than males, we hypothesized that this was primarily related to the higher PTSD rates reported by females than in males, with almost twice as much the levels. This appears to be in contrast with a previous research on civilian war victims, where women showed higher levels of somatization symptoms than men, whereas the levels of PTSD symptoms were similar across the two genders.Reference Morina, Schnyder and Klaghofer 69 However, a systematic review, examining the association of reported psychological trauma and PTSD with functional somatic syndromes, showed no significant gender effect on the prevalence of functional somatic syndromes in individuals who reported exposure to trauma, even a slightly but not significant higher rate in males.Reference Afari, Ahumada and Wright 37 In order to better clarify this issue, we performed a further analysis to examine the contribution of continuous PTSD symptoms on somatic symptoms above and beyond sex. Results showed re-experiencing and maladaptive coping TALS-SR domains to be the only related to the presence of at least one MOODS-SR somatic symptom. For what concern the re-experiencing symptoms, we argue that these symptoms are usually related to prolonged rumination on the trauma and its consequences. Szabo et alReference Szabo, Warnecke and Newton 70 showed a moderate, positive relationship between rumination and PTSD symptoms in trauma-exposed adults, with a stronger association between rumination and intrusive re-experiencing than avoidance or hyperarousal. Rumination is, in fact, defined as a form of perseverative cognition that focuses on negative content, generally past and present, and results in emotional distress.Reference Sansone and Sansone 71 Emerging literature suggests the possible role of rumination in fully mediating the relationship between neuroticism and somatic complaints.Reference Denovan, Dagnall and Lofthouse 72 Lohaus et al,Reference Lohaus, Vierhaus and Frevert 73 in a sample of children and adolescents, showed ruminative response style to be related to increased frequency of experiencing somatic and psychological symptoms, particularly among girls. Our results may account for a primary role of post-traumatic stress reactions on the relationship with somatic symptoms beyond gender. For what concern our results on the relationship between TALS-SR maladaptive coping and somatic symptoms, we may suggest this relationship may have a dual lapel. If on the one hand, maladaptive symptoms and self-destructive behaviors are fully acknowledged as core PTSD DSM-5 symptoms (within diagnostic criterion E), 1 , Reference Carmassi, Barberi and Cordone 74 our results may account for a relationship between an increased severity of the PTSD and the somatic complains. On the other hand, in the maladaptive coping domain, the TALS-SR items include “having stopped taking care of themselves” and “the use of drugs or over the counter medications to relief symptoms,” and this may be consequent to the presence of somatic symptoms per-se. Previous studies on trauma-exposed adolescent showed high rates of drugs, alcohol, or medication (such as painkillers) to calm themselves or to relieve emotional or physical pain.Reference Dell’Osso, Carmassi and Stratta 12 , Reference Carmassi, Rossi and Pedrinelli 13 , Reference Glodich and Allen 75 , Reference Steven, Murphy and McKnight 76
Some important limitations of the present study should be kept in mind when interpreting our results. The first limitation is the lack of information about psychiatric comorbidities in the sample, including prior psychiatric history; this could, in fact, have an impact on the development of PTSD or somatic symptoms. Second is the use of self-report instruments that could be considered less accurate than the rating of the clinician. Third is represented by the lack of information on the presence of somatic illness and on quality of life in the sample. Fourth is the fact that we did not use a specific scale to assessing chronic pain.
Conclusion
Despite these limitations, our results highlight the significant relationship between somatic symptoms developed in young adult population in the aftermath of a massive trauma exposure, such as an earthquake, suggesting a potential higher vulnerability in those subjects presenting PTSD without a clear prevalence of gender. Evaluating the burden of somatic symptoms seems essential for a more accurate assessment and clinical management of survivors to mass traumas, such as earthquakes or other natural catastrophic events, with post-traumatic stress symptoms, also in order to prevent inappropriate painkillers prescriptions. In light of our findings, in fact, treatment should focus on PTSD symptoms and on re-experiencing and rumination on the trauma and its consequence in order to provide adequate psychopharmacological and psychological treatment, to prevent somatizations and, potentially, the often-revealed abuse of painkillers and over the counter medications in such populations. However, scant trials are still available on effective treatments suggesting the need for further research in this specific field.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Disclosures
The authors report no conflicts of interest.








